Abstract
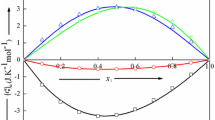
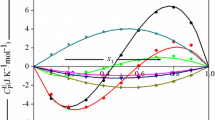
Densities and heat capacities of ternary systems were determined at 25°C. The ternary systems consisted of: a polar molecule (component 1) + a mixture of alkanes (components 2 and 3) of different sizes and shapes. Five such systems were studied: chlorobenzene + cyclohexane + n-heptane; chlrobenzene + cyclohexane + n-hexadecane; chlorobenze + cyclohexane + isooctane; chlorobenzene + isooctane + n-heptane; 1-chloronaphthalene + isooctane + n-heptane. The excess molar volumes and heat capacities were obtained along dilution lines by component 1 (chlorobenzene or 1-chloronaphthalene) of mixtures of components 2 and 3 (at fixed component 2 mole fraction X2). Unexpectedly the excess heat capacities C Ep1(23) of the pseudo-binaries {1+(2+3)} do not always fall between the two (limiting) curves of C Ep12 and C Ep13 corresponding to the two binaries {1+2} and {1+3}. Instead, especially for {chlorobenzene + cyclohexane + an n-alkane} the C Ep1(23) curves are displaced toward less negative values, even beyond the limiting values corresponding to the binaries. This correlates semi-quantitatively with the negative C Ep23 of the binary {2+3}.
Similar content being viewed by others
References
S. N. Bhattacharyya, M. Costas, D. Patterson, and H. V. Tra,Fluid Phase Equilibria 20, 27, (1985).
A. Heintz and R. N. Lichtenthaler,Angew. Chem. Int. Ed. Engl. 21, 184 (1982)
J.-P. E. Grolier, A. Inglese, A.H. Roux, and E. Wilhelm,Chem. Eng. Thermodyn., Vol. 4, S. A. Newman, ed., (Ann Arbor Science, Ann Arbor, 1982). p. 83.
P. Picker, E. Tremblay, and C. Jolicoeur,J. Solution Chem.,3, 377 (1974).
P. Picker, P. A. Leduc, P. R. Philip, and J. E. Desnoyers,J. Chem. Thermodyn. 3, 631 (1971)
J.-P. E. Grolier, G. C. Benson and P. Picker,J. Chem. Eng. Data,20, 243 (1975).
J. A. Riddick, W. B. Bunger, and T. K. Sakano,Organic Solvents, Physical Properties and Methods of Purification, 4th edn., (Wiley, New York, 1986).
J. L. Fortier and G. C. Benson,J. Chem. Thermodyn. 8, 289 (1976).
S. Perez-Casas, E. Aicart, L. M. Trejo and M. Costas,Int. Data Ser. Sel. Data Mixtures, Ser. A 2, 119, (1988).
E. Wilhelm, A. Lainez, A. H. Roux, and J-P. E. Grolier,Thermochimica Acta 105, 101, (1986).
M. Costas, H. V. Tra, D. Patterson, M. Caceres-Alonso, G. Tardajos, and E. Aicart,J. Chem. Soc. Faraday Trans. I 84, 1603, (1988).
A. Saito and R. Tanaka,J. Chem. Thermodyn. 20, 859 (1988).
S. N. Bhattacharyya and D. Patterson,J. Phys. Chem. 83, 2979, (1979).
J-P.E. Grolier, A. Inglese, A.H. Roux, and E. WilhelmBer. Bunsenges. Phys. Chem. 85, 768, (1981).
A. Dondos and D. Patterson,J. Polymer Sci. A2,5, 230, (1967).
G. Delmas, D. Patterson, and S. N. Battacharryya,J. Phys. Chem. 68, 1468, (1964).
L. Andreoli-Ball, M. Costas, D. Patterson, R. G. Rubio, R. M. Masegosa, and M. Caceres,Ber. Bunsenges. Phys. Chem. 93, 882, (1989).
J-P. E. Grolier, A. Inglese and E. Wilhelm,J. Chem. Thermodyn. 16, 67, (1984).
E. Jimenez, L. Romani, M. I. Paz Andrade, G. Roux-Desgranges and J-P. E. Grolier,J. Solution Chem.,15, 879, (1986):
M. Pintos, R. Bravo, M. C. Baluja, M. I. Paz Andrade, G. Roux-Desgranges and J-P. E. Grolier,Can. J. Chem. 66, 1179, (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bendiab, H., Roux-Desgranges, G., Roux, A.H. et al. Excess heat capacities of ternary systems containing chlorobenzene or chloronaphthalene. J Solution Chem 23, 307–323 (1994). https://doi.org/10.1007/BF00973552
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00973552