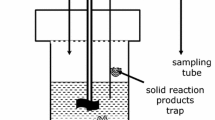
Abstract
A platinum-lined flowing autocláve facility was used to investigate the solubility behavior of magnetite (Fe3O4) in alkaline sodium phosphate and ammonium hydroxide solutions between 21 and 288°C. Measured iron solubilities were interpreted via a Fe(II)/Fe(III) ion hydroxo-, phosphato-, and ammino-complexing model and thermodynamic functions for these equilibria were obtained from a least-squares analysis of the data. A total of 14 iron ion species were fitted. Complexing equilibria are reported for 8 new species: Fe(OH)(HPO4)−, Fe(OH)2(HPO4)2−, Fe(OH)3(HPO4)2−, Fe(OH)(NH3)+, Fe(OH)2(PO4)3−, Fe(OH)4(HPO4)3−, Fe(OH)2(H2PO4)−, and Fe(OH)3(H2PO4)3−. At elevated temperatures, hydrolysis and phosphato complexing tended to stabilize Fe(III) relative to Fe(II), as evidenced by free energy changes fitted to the oxidation reactions.
For temperatures below 83°C and for a dissolved hydrogen concentration of 234 μmol-kg−1, the activity of ferrous iron in aqueous solution is controlled by a hydrous Fe(II) oxide solid phase rather than magnetite.
Similar content being viewed by others
References
F. H. Sweeton and C. F. Baes,J. Chem. Thermodyn. 2, 479 (1970).
P. R. Tremaine and J. C. LeBlanc,J. Solution Chem. 9, 415 (1980).
S. E. Ziemniak, M. E. Jones and K. E. S. Combs,J. Solution Chem. 18, 1133 (1989).
S. E. Ziemniak and E. P. Opalka, inProc. Sixth International Symposium on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, R. E. Gold and E. P. Simonen, eds., (The Minerals, Metals & Materials Society, Warrendale, PA, 1993), p. 929.
E. C. Potter and G. M. W. Mann, inProceedings of the First International Congress on Metallic Corrosion, (Butterworths, London, 1962), p. 417.
E. M. Field and D. R. Holmes,Corros. Sci. 5 361 (1965).
Private communication with J. Chera, GE Corporate R&D Center.
N. S. McIntyre and D. G. Zetaruk,Anal. Chem. 49, 1521 (1977).
J. O. Nriagu,Geochim. Cosmochim. Acta. 37, 2357 (1972).
H. Galal-Gorchev and W. Stumm,J. Inorg. Nucl. Chem. 25, 567 (1963).
K. B. Yatsimirskii,J. Gen. Chem. USSR 24, 1485 (1954).
F. H. Sweeton, R. E. Mesmer, and C. F. Baes,J. Solution Chem. 3, 191 (1974).
B. F. Hitch and R. E. Mesmer,J. Solution Chem. 5, 667 (1976).
R. E. Mesmer and C. F. Baes,J. Solution Chem. 3, 307 (1974).
N. C. Treloar,Central Electricity Research Laboratory Report RD/L/N 270/73 (1973). (See WAPD-TM-1302, March 1979).
L. O. Gilpatrick and H. H. Stone,Oak Ridge National Laboratory Reports ORNL-3127 (1961) and ORNL-3262 (1962).
W. L. Marshall, R. Slusher, and E. V. Jones,J. Chem. Eng. Data 9, 187 (1964).
W. L. Marshall and E. V. Jones,J. Phys. Chem. 70, 4028 (1966).
D. L. Marquardt,J. Soc. Indust. Appl. Math. 2, 431 (1963).
C. F. Baes and R. E. Mesmer,The Hydrolysis of Cations Wiley-Interscience, New York, (1976).
K. H. Gayer and A. B. Garrett,J. Amer. Chem. Soc. 72, 3921 (1950).
J. W. Larson, P. Cerutti, H. K. Garber and L. G. Hepler,J. Phys. Chem. 72, 2902 (1968).
D. D. Wagman, W. H. Evans, V. B. Parker, R. H. Schumm, I. Halow, S. M. Bailey, K. L. Churney, and R. L. Nuttall, “The NBS Tables of Chemical Thermodynamic Properties”,J. Phys. Chem. Ref. Data 11, Suppl. No. 2 (1982).
M. M. Osman, T. M. Salem, and N. J. L. Gayed,Inorg. Chimica Acta 58, 233 (1981).
G. K. Johnson and J. E. Bauman,Inorg. Chem. 17, 2774 (1978).
I. Barin,Thermochemical Data of Pure Substances, (VCH Verlagsgesellschaft, Weinheim, 1989).
M. H. Abraham and Y. Marcus,J. Chem. Soc., Faraday Trans. 1,82, 3255 (1986).
R. E. Mesmer,Inorg. Chem. 10, 857 (1971).
G. A. Kanert, G. W. Gray, and W. G. Baldwin,Report AECL-5528 (Atomic Energy of Canada, Ottawa, 1976).
M. A. Styrikovich, O. I. Martynova, I. F. Kobyakov, V. L. Men'shikova, and M. I. Reznikov,Therm. Eng. 19, 127 (1972).
I. Lambert, J. Montel, P. Beslu, and A. Lalet, inThermodynamics of Nuclear Materials 1979, (International Atomic Energy Agency, Vienna, 1980) p. 89.
I. Lambert, J. Montel, and P. Courvoisier, inProc. Second International Conference on Water Chemistry of Nuclear Reactor Systems, (British Nuclear Energy Society, London, 1980), p. 31.
S. C. Lahiri,J. Ind. Chem. Soc. 42, 715 (1965).
J. O. Nriagu,Amer. J. Sci. 272, 476 (1972).
R. F. Schmalz,J. Geophys. Res.,64, 575 (1959).
C. M. Criss and J. W. Cobble,J. Amer. Chem. Soc. 86, 5390 (1964).
J. W. Larson, K. G. Zeeb, and L. G. Hepler,Can. J. Chem. 60, 2141 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ziemniak, S.E., Jones, M.E. & Combs, K.E.S. Magnetite solubility and phase stability in alkaline media at elevated temperatures. J Solution Chem 24, 837–877 (1995). https://doi.org/10.1007/BF00973442
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00973442