Abstract
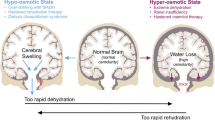
Diffusion-weighted in vivo1H-NMR spectroscopy of F98 glioma cells embedded in basement membrane gel threads showed that the initial cell swelling to about 180% of the original volume induced under hypotonic stress was followed by a regulatory volume decrease to nearly 100% of the control volume in Dulbecco's modified Eagle's medium (DMEM) but only to 130% in Krebs-Henseleit buffer (KHB, containing only glucose as a substrate) after 7 h. The initial cell shrinkage to approx. 70% induced by the hypertonic stress was compensated by a regulatory volume increase which after 7 h reached almost 100% of the control value in KHB and 75% in DMEM.1H-,13C-and31P-NMR spectroscopy of perchloric acid extracts showed that these volume regulatory processes were accompanied by pronounced changes in the content of organic osmolytes. Adaptation of intra- to extracellular osmolarity was preferentially mediated by a decrease in the cytosolic taurine level under hypotonic stress and by an intracellular accumulation of amino acids under hypertonic stress. If these solutes were not available in sufficient quantities (as in KHB), the osmolarity of the cytosol was increasingly modified by biosynthesis of products and intermediates of essential metabolic pathways, such as alanine, glutamate and glycerophosphocholine in addition to ethanolamine. The cellular nucleoside triphosphate level measured by in vivo31P-NMR spectroscopy indicated that the energy state of the cells was more easily sustained under hypotonic than hypertonic conditions.
Similar content being viewed by others
References
Chamberlain, M. E., and Strange, K. 1989. Anisosmotic cell volume regulation: a comparative view. Am. J. Physiol. 257:C159-C173.
Hoffmann, E. K., and Simonson, L. O. 1989. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol. Rev. 69:315–382.
Lang, F., Ritter, M., Völkl, H., and Häussinger, D. 1993. Cell volume regulatory mechanims—an overview, Pages 1–32,in Lang, F., and Häussinger, D. (eds.), Adv. Comp. Environ. Physiol. Vol. 14, Springer-Verlag, Berlin.
Syková, E. 1992. Ionic and volume changes in the microenvironment of nerve and receptor cells. Progr. Sens. Physiol. 13:1–167.
Cserr, H. F., and Patlak, C. S. 1991. Regulation of brain volume under isosmotic and anisosmotic conditions, Pages 61–80,in Gilles, R., Hoffmann, E. K., and Bolis, L. (eds.), Adv. Comp. Environ. Physiol. Vol. 9. Springer-Verlag, Berlin.
Kimelberg, H. K., O'Connor, E. R., and Kettenmann, H. 1993. Effects of swelling on glial cell function, Pages 157–186,in Lang, F. and Häussinger, D. (eds.), Adv. Comp. Environ. Physiol. Vol. 14, Springer-Verlag, Berlin.
Pollock, A. S., and Arieff, A. I. 1980: Abnormalities of cell volume regulation and their functional consequences. Am. J. Physiol. 239:F195-F205.
Flynn, C. J., Farooqui, A. A., and Horrocks, L. A. 1994. Ischemia and hypoxia, Pages 783–795,in Siegel, G. J. Agranoff, B. W., Albers, R. W., and Molinoff, P. B. (eds.), Basic Neurochemistry: Molecular, Cellular, and Medical Aspects, 5th Ed., Raven Press, New York.
Jakubovicz, D. E., Grinstein, S., and Klip, A. 1987. Cell swelling following recovery from acidification in C6 glioma cells: an in vitro model of post-ischemic brain edema. Brain Res. 435:138–146.
Walz, W., Klimaszweski, A., and Paterson, I. A. 1993. Glial swelling in ischemia: A hypothesis. Dev. Neurosci. 15:216–225.
Kinne, R. K. H. 1993. The role of organic osmolytes in osmoregulation: from bacteria to mammals. J. Exp. Zool. 265:346–355.
Law, R. O., and Burg, M. B. 1991. The role of organic osmolytes in the regulation of mammalian cell volume, Pages 189–225,in Gilles, R., Hoffmann, E. K., and Bolis, L. (eds.), Adv. Comp. Environ. Physiol. Vol. 9. Springer-Verlag, Berlin.
McCarthy, N. A., and O'Neil, R. G. 1992. Calcium signaling in cell volume regulation. Physiol. Rev. 72:1037–1061.
Burg, M. B. 1994. Molecular basis for osmoregulation of organic osmolytes in renal medullary cells. J. Exp. Zool. 268:171–175.
Heilig, C. W., Brenner, R. M., Yu, A. S. L., Kone, B. C., and Gullans, S. R. 1990. Modulation of osmolytes in MDCK cells by solutes, inhibitors, and vasopressin. Am. J. Physiol. 259:F653–659.
Lohr, J. W., and Grantham, J. J. 1991. Inorganic ions and volume regulation in kidney tubules under anisosmotic conditions, Pages 61–80,in Gilles, R., Hoffmann, E. K., and Bolis, L. (eds.), Adv. Comp. Environ. Physiol. Vol. 9. Springer-Verlag, Berlin.
Zablocki, K., Miller, S. P. F., Garcia-Perez, A., and Burg, M. B. 1991. Accumulation of glycerophosphocholine (GPC) by renal cells: Osmotic regulation of GPC: choline phosphodiesterase. Proc. Natl. Acad. Sci. U.S.A. 88:7820–7824.
Beetsch, J. W., and Olson, J. E. 1993. Taurine transport in rat astrocytes adapted to hyperosmotic stress. Brain Res. 613:10–15.
Kimelberg, H. K. 1991. Swelling and volume control in brain astroglial cells, Pages 81–117,in Gilles, R., Hoffmann, E. K., and Bolis, L. (eds.), Adv. Comp. Environ. Physiol. Vol. 9. Springer-Verlag, Berlin.
Law, R. O. 1994. Regulation of mammalian brain cell volume. J. Exp. Zool. 268:90–96.
Strange, K., and Morrison, R. 1992. Volume regulation during recovery from chronic hypertonicity in brain glial cells. Am. J. Physiol. 263:C412-C419.
Heilig, C. W., Stromski, M. E., Blumenfeld, J. D., Lee, J. P., and Gullans, S. R. 1989. Characterization of the major brain osmolytes that accumulate in salt-loaded rats. Am. J. Physiol. 257:F1108-F1116.
Lien, Y. H. H., Shapiro, J. I., and Chan, L. 1990. Effects of hypernatremia on organic brain osmoles. J. Clin. Invest. 85:1427–1435.
Law, R. O. 1994. Taurine efflux and the regulation of cell volume in incubated slices of rat cerebral cortex. Biochim. Biophys. Acta 1221:21–28.
Pasantes-Morales, H., and Schousboe, A. 1988. Volume regulation in astrocytes: a role for taurine as osmoeffector. J. Neurosci. Res. 20:505–509.
Solis, J. M., Herranz, A. S., Herreras, O., Lerma, J., and Martin del Rio, M. 1988. Does taurine act as an osmoregulatory substance in rat brain? Neurosci. Lett. 91:53–58.
Wade, J. V., Olson, J. P., Samson, F. E., Nelson, S. R., and Pazdernik, T. L. 1988. A possible role for taurine in osmoregulation within the brain. J. Neurochem. 51:740–745.
Isaacks, R. E., Bender, A. S., Kim, C. Y., Prieto, N. M., and Norenberg, M. D. 1994. Osmotic regulation of myo-inositol uptake in primary astrocyte cultures. Neurochem. Res. 19:331–338.
Strange, K., Morrsion, R., Shrode, L., and Putnam, R. 1993. Mechanism and regulation of swelling-activated inositol efflux in brain glial cells. Am. J. Physiol. 265:C244-C256.
Verbalis, J. G., and Gullans, S. R. 1991. Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res. 567:274–282.
Fishman, R. A., Reiner, M., and Chan, P. H. 1977. Metabolic changes associated with iso-osmotic regulation in brain cortex slices. J. Neurochem. 28:1061–1067.
Häussinger, D., Gerok, W., and Lang, F., 1993. Cell volume and hepatic metabolism, Pages 33–66,in Lang, F., and Häussinger, D., (eds.), Adv. Comp. Environ. Physiol. Vol. 14, Springer-Verlag, Berlin.
Olson, J. E., Evers, J. A., and Holtzman, D. 1992. Astrocyte volume regulation and ATP and phosphocreatine concentrations after exposure to salicylate, ammonium, and fatty acids. Metab. Brain Dis. 7:183–196.
Ko, L., Koestner, A., and Wechsler, W. 1980. Morphogical characterization of nitrosurea-induced glioma cell lines and clones. Acta Neuropathol. 51:23–31.
Ko, L., Koestner, A., and Wechsler, W. 1980. Characterization of cell cycle and biological parameters of transplantable glioma cell lines and clones. Acta Neuropathol. 51:107–111.
Daly, P. F., Lyon, R. C., Straka, E. J., and Cohen, J. S. 1988.31P-NMR of human cancer cells proliferating in a basement membrane gel. FASEB J. 2:2596–2604.
Goa, J. 1953. A micro biuret method for protein determination. Scand. J. Clin. Lab. Invest. 5:218–222.
Shaka, A. J., Keeler, J., Frenkiel, T., and Freeman, R. 1983. An improved sequence for broadband decoupling: WALTZ-16. J. Magn. Reson. 52:335–338.
Gillies, R. J., Alger, R. J., den Hollander, J. A., and Shulman, R. G. 1982. Intracellular pH measured by NMR: methods and results, Pages 79–104,in Nuccitelli, R., and Deamer, D. W. (eds.), Intracellular pH: its Measurement, Regulation, and Utilization in Cellular Functions, Alan R. Liss, Inc., New York.
Fabry, M. E., and San George, R. C., 1983. Effect of magnetic susceptibility on nuclear magnetic resonance signals arising from red cells: a warning. Biochemistry 22:4119–4125.
Neeman, M., Jarrett, K. A., Sillerud, L. O., and Freyer, J. P. 1991. Self-diffusion of water in multicellular spheroids measured by magnetic resonance microimaging. Canc. Res. 51:4072–4079.
van Zijl, P. C. M., Monnen, C. T. W., Faustino, P., Pekar, J., Kaplan, O., and Cohen, J. S. 1991. Complete separation of intracellular and extracellular information in NMR spectra of perfused cells by diffusion-weighted spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 88:3228–3232.
Niendorf, T., Flögel, U., Norris, D. G., and Leibfritz, D. 1993. Intracellular water diffusion in glioma and neuroblastoma cells, Page 600,in Book of Abstracts, 12th Annual Meeting, Society of Magnetic Resonance, New York.
Stejskal, E. O., and Tanner, J. E. 1988. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 42:288–292.
Halliday, K. R., Fenoglio-Preiser, C., and Sillerud, L. O. 1988. Differentiation of human tumors from nonmalignant tissue by natural-abundance13C-NMR spectroscopy. Magn. Reson. Med. 7: 384–411.
Hamilton, J. A., and Morisett, J. D. 1986. Nuclear magnetic resonance studies of lipoproteins. Methods Enzymol. 128:472–515.
Ribeiro, A. A., and Dennis, E. A. 1976.13C nuclear magnetic resonance relaxation studies on the structure of mixed micelles of the nonionic surfactant Triton X-100 and phospholipids. J. Colloid Interface Sci. 55:94–101.
Pasantes-Morales, H., Alavez, S., Olea, R. S., and Morán, J. 1993. Contribution of organic and inorganic osmolytes to volume regulation in rat brain cells in culture. Neurochem. Res. 18:445–452.
Lien, Y. H. H., Zhou, H. Z., Job, C., Barry, J. A., and Gillies, R. J. 1992. In vivo NMR study of early cellular responses to hyperosmotic shock in cultured glioma cells. Biochimie 74:931–939.
Natke, E. 1990. Cell volume regulation of rabbit cortical collecting tubule in anisotonic media. Am. J. Physiol. 258:F1657-F1665.
Rome, L., Grantham, J., Savin, V., Lohr, J., and Lechene, C. 1989. Proximal tubule volume regulation in hyperosmotic media: intracellular K+, Na+, and Cl−. Am. J. Physiol. 257:C1093-C1100.
Tyler, B. C., and Ribolow, H. 1994. Glycerol phosphorylcholine (GPC) and serine ethanolamine phosphodiester (SEP): evolutionary mirrored metabolites and their potential metabolic roles. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 108:11–20.
Lang, F., Busch, G., Völkl, H., and Häussinger, D. 1994. Lysosomal pH: a link between cell volume and metabolism. Biochem. Soc. Trans. 22:502–505.
Meijer, A. J., Gustafson, L. A., Luiken, J. J. F. P., Blommaart, P. J. E., Caro, H. P., van Woerkom, G. M., Spronk, C., and Boon, L. 1993. Cell swelling and the sensitivity of autophagic proteolysis to inhibition by amino acids in isolated rat hepatocytes. Eur. J. Biochem. 215:449–454.
Bauernschmitt, H. G., and Kinne, R. K. H. 1993. Metabolism of the ‘organic osmolyte’ glycerophosphocholine in isolated rat inner medullary collecting duct cells. II. Regulation by extracellular osmolality. Biochim. Biophys. Acta 1150:25–34.
Cala, P., and Grinstein, S. 1988. Coupling between Na+/H+ and Cl−/HCO − 3 exchange in pH and volume regulation; Pages 201–208,in Grinstein, S. (ed.), Na+/H+ Exchange, CRC Press, Boca Raton.
Lien, Y. H. H., Zhou, H. Z., Lai, L. W., Job, C., and Gillies, R. J. 1993. NMR study of regulatory volume increase in cultured glioma cells: role of Na+/H+-antiporter, Page 1013,in Book of Abstracts, 12th Annual Meeting, Society of Magnetic Resonance, New York.
Parker, C. 1988. Na+/H+ exchange and volume regulation in nonepithelial cells, Pages 179–200in Grinstein S. (ed.), Na+/H+ exchange, CRC Press, Boca Raton.
Munsch, T., and Deitmer, J. W. 1994. Sodium-bicarbonate cotransport current in identified leech glial cells. J. Physiol. (London) 474:43–53.
Author information
Authors and Affiliations
Additional information
To whom to address reprint requests.
Rights and permissions
About this article
Cite this article
Flögel, U., Niendorf, T., Serkowa, N. et al. Changes in organic solutes, volume, energy state, and metabolism associated with osmotic stress in a glial cell line: A multinuclear NMR study. Neurochem Res 20, 793–802 (1995). https://doi.org/10.1007/BF00969691
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00969691