Abstract
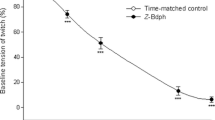
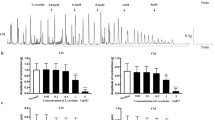
The toxic effect of mercuric ions on intestinal cholinergic neurotransmission was investigated in vitro. Hg2+ inhibited the evoked release and enhanced the resting release of ACh. Smooth muscle contraction was irreversibly inhibited by Hg2+ in a concentration-dependent manner, and Na2EDTA did not antagonize this effect. We also investigated if Hg2+ enters the nerve terminal through Ca2+-channels, or Na+-channels, or both. The effects of mercuric ions obtained in our study were completely abolished by the combined administration of TTX and Co2+. It is suggested that the site of the action of mercuric ions is intracellular. We concluded that Hg2+ may interfere with cholinergic transmission by blocking [Ca2+]o-dependent release of ACh and by enhancing [Ca2+]o-independent resting release of ACh. The effect of Hg2+ was not only presynaptic since it also inhibited the effect of ACh on smooth muscle.
Similar content being viewed by others
References
Goodman and Gilman's 1990. The pharmacological basis of therapeutics. A. Goodman Gilman, T. W. Rall, A. S. Nies, P. Taylor (eds.), Pergamon Press, Inc.
Fölkl, R. 1986. Munkaegeszsegugyi es munkavedelmi enciklopedia. Budapest, OMIKK, Pages 426–429, 1074–1081. (in Hungarian)
Venugopal, B., and Luckey, T. P. 1975. Toxicology of non-radioactive heavy metals and their salts. Environ. Qual. Saf. Suppl. 1:4–73.
Paton, W. D. M., and Vizi, E. S. 1969. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guineapig ileum longitudinal muscle strip. Br. J. Pharmacol. 35:10–28.
Paton, W. D., and Zar, M. A., 1968. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J. Physiol. (London) 194:13–33.
Paton, W. D. M., Vizi, E. S., and Zar, M. A. 1971. The mechanism of acetylcholine release from parasympathetic nerves. J. Physiol. (London) 215:819–848.
Somogyi, G. T. and Vizi, E. S. 1988. Evidence that cholinergic axon terminals are equipped with both muscarinic and adenosine receptors. Brain Res. Bull. 21:575–579.
Kilbinger, H., and Wessler, I. 1980. Inhibition by acetylcholine of the stimulation-evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus. Neuroscience 5:1331–1340.
Vizi, E. S., Ono, K., Adam-Vizi, V., and Foldes, F. F. 1984. Presynaptic inhibitory effect of Met-enkephalin on 14C-acetylcholine release from the myenteric plexus and its interaction with muscarinic negative feedback inhibition. J. Pharmacol. Exp. Ther. 230:493–499.
Vizi, E. S., Torok, T., Seregi, A., Serfozo, P., and Adam-Vizi, V. 1982. Na−K-activated ATPase and the release of acetylcholine and noradrenaline. J. Physiol. (Paris) 78:399–406.
Vizi, E. S., and Bernath, S. 1987. Inhibitory effect of ionized free intracellular calcium enhanced by ruthenium red and m-chlorocarbonylcyanide phenyl hydrazon on the evoked release of acetylcholine. Biochem. Pharmacol. 36:3683–3687.
Vizi, E. S. 1978. Na+, K+-activated adenosinetriphosphatase as a trigger in transmitter release. Neuroscience 3:367–384.
Vizi, E. S., Oberfrank, F. 1992. Na+/K+-ATPase, its endogenous ligands and neurotransmitter release. Neurochem. Int. 20:11–17.
Juang, M. S. 1976. An electrophysiological study of the action of methylmercuric chloride and mercuric chloride on the sciatic nerve-sartorius muscle preparation of the frog. Toxicol. Appl. Pharmacol. 37:339–348.
Manalis, R. S., and Cooper, G. P. 1975. Evoked transmitter release increased by inorganic mercury at frog neuromuscular junction. Nature 257:690–691.
Miyamoto, M. D. 1983. Hg2+ causes neurotoxicity at an intracellular site following entry through Na and Ca channels. Brain Res. 267:375–379.
Atchison, W. D. 1987. Effects of activation of sodium and calcium entry on spontaneous release of acetylcholine induced by methylmercury. J. Pharmacol. Exp. Ther. 241:131–139.
Atchison, W. D. 1988. Effects of neurotoxicants on synaptic transmission: lessons learned from electrophysiological studies. Neurotoxicol.-Teratol. 10:393–416.
Levesque, P. C., Hare, M. F., and Atchison, W. D. 1992. Inhibition of mitochondrial Ca2+ release diminishes the effectiveness of methyl mercury to release acetylcholine from synaptosomes. Tox. Appl. Pharmacol. 115:11–20.
Denny, M. F., Hare, M. H., and Atchison, W. D. 1993. Methylmercury alters intrasynaptosomal concentrations of endogenous polyvalent cations. Toxicol. Appl. Pharmacol. 122:222–232.
Rossi, A. D., Larsson, O., Manzo, L., Orrenins, S., Vahler, M., Berggren, P.-O., and Nicotera, P. 1993. Modification of Ca2+ signaling by inorganic mercury in PC12 cells. FASEB J. 7:1507–1514.
Vizi, E. S., Bernath, S., Kapocsi, J., and Serfozo, P. 1986. Transmitter release from the cytoplasm is of physiological importance but not subject to presynaptic modulation. J. Physiol. (Paris) 81:283–288.
Dolezal, V., Somogyi, G. T., Bernath, S., Tucek, S., and Vizi, E. S. 1987. Effect of lanthanum on the release of acetylcholine from the myenteric plexus and on its activation by ouabain and electrical stimulation. J. Neurochem. 49:503–506.
Adam-Vizi, V. 1992. External Ca2+-independent release of neurotransmitter. J. Neurochem. 58:395–405.
Lees, G. J. 1991. Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res. Rev. 16:283–300.
Vizi, E. S. 1972. Stimulation by inhibition of (Na+,K+,Mg2+)-activated ATPase, of acetylcholine release in cortical slices from rat brain. J. Physiol. (Lond.) 226:95–117.
Anner, R., and Moormayer, M. 1992. Mercury block Na−K ATPase by a ligand-dependent and reversible mechanism. Am. J. Physiol. 262:F830-F836.
Anner, R., and Moormayer, M. 1992. Mercury inhibits Na/K-ATPase primarily at the cytoplasmic side. Am. J. Physiol. 262:F843-F848.
Godfraind, M. 1986. Calcium antagonist and calcium entry blockade. Pharmacol. Rev. 38:321–416.
Hurwitz, L. 1986. Pharmacology of calcium channels and smooth muscle. Annu. Rev. Pharmacol. Toxicol. 26:225–258.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abram, Z., Korossy, S. Presynaptic and postsynaptic effects of mercuric ions on guinea-pig ileum longitudinal muscle strip preparation. Neurochem Res 19, 1467–1472 (1994). https://doi.org/10.1007/BF00968992
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00968992