Abstract
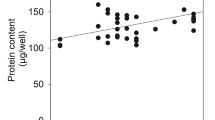
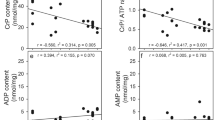
Astrocytes are important in regulating the microencironment of neurons both by catabolic and synthetic pathways. The glutamine synthetase (GS) activity observed in astrocytes affects neurons by removing toxic substances, NH3 and glutamate; and by providing an important neuronal substrate, glutamine. This glutamate cycle might play a critical role during periods of hypoxia and ischemia, when an increase in extracellular excitatory amino acids is observed. It was previously shown in our laboratory that fructose-1,6-bisphosphate (FBP) protected cortical astrocyte cultures from hypoxic insult and reduced ATP loss following a prolonged (18–30 hrs) hypoxia. In the present study we established the effects of FBP on the level of glutamate uptake and GS activity under normoxic and hypoxic conditions. Under normoxic conditions, [U-14C]glutamate uptake and glutamine production were independent of FBP treatment; whereas under hypoxic conditions, the initial increase in glutamate uptake and an overall increase in glutamine production in astrocytes were FBP-dependent. Glutamine synthetase activity was dependent on FBP added during the 22 hours of either normoxic- or hypoxic-treatment, hence significant increases in activity were observed due to FBP regardless of the oxygen/ATP levels in situ. These studies suggest that activation of GS by FBP may provide astrocytic protection against hypoxic injury.
Similar content being viewed by others
References
Martinez-Hemandez, A., Bell, K. P., and Norenberg, M. D. 1977. Glutamine synthetase: Glial localization in brain. Science 195:1356–1358.
Norenberg, M. D., and Martinez-Hernandez, A. 1979. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 161:303–310.
Tansey, F. A., Farooq, M., and Cammer, W. 1991. Glutamine synthetase in oligodendrocytes and astrocytes: new biochemical and immunocytochemical evidence. J. Neurochem. 56:266–272.
D'Amelio, F., Eng, L. F., and Gibbs, M. A. 1990. Glutamine synthetase immunoreactivity is present in oligodendroglia of various regions of the central nervous system. Glia 3:335–341.
Benjamin, A. M., and Quastel, J. H. 1972. Locations of amino acids in brain cortex slices from the rat. Tetrodotoxin-sensitive release of amino acids. Biochem. J. 128:631–646.
Benjamin, A. M., and Quastel, J. H. 1975. Metabolism of amino acids and ammonia in rat brain cortex slices in vitro. A possible role of ammonia in brain function. J. Neurochem. 25:197–206.
Waniewski, R. A., and Martin, D. L. 1986. Exogenous glutamate is metabolized to glutamine and exported by rat primary astrocyte cultures. J. Neurochem. 47:304–313.
Yu, A. C. H., Fisher, T. E., Hertz, E., Tildon, J. T., Schousboe, A., and Hertz, L. 1984. Metabolic fate of [14C]-glutamine in mouse cerebral neurons in primary cultures. J. Neurosci. Res. 11:351–357.
Shank, R. P., and Campbell, G. L. 1983. Metabolic precursors of glutamate and GABA. Pages 355–369,in Hertz, L., Kvamme, E., McGeer, E. G., and Schousboe, A. (eds.), Glutamine, Glutamate and GABA in the Central Nervous System, Alan R. Liss, New York.
Paulson, R. E., and Fonnum, F. 1989. Role of glial cells for the basal and Ca2+-dependent K+-evoked release of transmitter amino acids investigated by microdialysis. J. Neurochem. 52(6):1823–1829.
Hertz, L., Bock, E., and Schousboe, A. 1978. GFA content, glutamate uptake and activity of glutamate metabolizing enzymes in differentiating mouse astrocytes in primary cultures. Dev. Neurosci. 1:226–238.
Patel, A. J., and Hunt, A. 1985. Observations on cell growth and regulation of glutamine synthetase by dexamethasone in primary cultures of forebrain and cerebellar astrocytes. Devl. Brain Res. 18:175–184.
Pishak, M. R., and Phillips, A. T. 1980. Glucocorticoid stimulation of glutamine synthetase production in cultured rat glioma cells. J. Neurochem. 34:866–872.
Aizenman, Y., and DeVellis, J. 1987. Synergistic action of thyroid hormone, insulin, and hydrocortisone on astrocyte differentiation. Brain Res. 414:301–308.
Benjamin, A. M. 1987. Influence of Na+, K+, and Ca2+ on glutamine synthesis distribution in rat brain cortex slices: A possible linkage of glutamine synthetase with cerebral transport processes and energetics in the astrocytes. J. Neurochem. 48:1157–1164.
Rothman, S. 1984. Synaptic release of excitatory amino acid neurotransmitter mediates anoxic neuronal death. J. Neurosci. 4:1884–1891.
Drejer, J., Benveniste, H., Diemer, N. H. and Schousboe, A. 1985. Cellular origin of ischemia-induced glutamate release from brain tissue in vivo and vitro. J. Neurochem. 45:145–151.
Nicholls, D. G., and Sihra, T. S. 1986. Synaptosomes possess an exocytotic pool of glutamat. Nature 321(6072):772–773.
Yu, A. C. H., Gregory, G. A., and Chan, P. H. 1989. Hypoxia-induced dysfunctions and injury of astrocytes in primary cell cultures. J. Cereb. Blood Flow & Metab. 9:20–28.
Gregory, G. A., Yu, A. C. H., and Chan, P. H. 1989. Fructose-1, 6-bisphosphate protects astrocytes from hypoxic damage. J. Cereb. Blood Flow & Metab. 9:29–34.
Gregory, G. A., Welsh, F. A., Yu, A. C. H., and Chan, P. H. 1990. Fructose-1,6-bisphosphate reduces ATP loss from hypoxic astrocytes. Brain Res. 516:310–312.
Farias, L. A., Smith, E. E., and Markov, A. K. 1990. Prevention of ischemic-hypoxic brain injury and death in rabbits with fructose-1,6-diphosphate. Stroke 21:606–613.
Booher, H., and Sensenbrenner, M. 1972. Growth and cultivation of dissociated neurons and glial cells from embryonic chick, and rat brain in flask cultures. Neurobiology 2:97–105.
Yu, A. C. H., Chan, P. H., and Fishman, R. A. 1986. Effects of arachidonic acid on glutamate and γ-aminobutyric acid uptake in primary cultures of rat cerebral astrocytes and neurons. J. Neurochem. 47:1181–1189.
Hertz, L., Juurlink, B. H. J., and Szuchet, S. 1985. Cell cultures. Pages 603–666,in Lajtha, A. (eds.) Handbook of Neurochemistry, Volume 8, Edition 2nd, Plenum Press, New York.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurements with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Pishak, M. R., and Phillips, A. T. 1979. A modified radioisotopic assay for measuring glutamine synthetase activity in tissue extracts. Anal. Biochem. 94:82–88.
Kauppinen, R. A., and Nicholls, D. G. 1986. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycemia. Eur. J. Biochem. 158:159–165.
Huang, H. M., Toral-Barza, L., and Gibson, G. 1991. Cytolsolic free calcium and ATP in synaptosomes after ischemia. Life Sciences 48(15):1439–145.
Simon, R. P., Swan, J. H., Griffiths, T., and Meldrum, B. S. 1984. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science 226:850–852.
Waniewski, R., and Martin, D. L. 1984. Characterization ofl-glutamic acid transport by glioma cells in culture: evidence for sodium-independent, chloride-dependent high affinity influx. J. Neurosci. 4(9):2237–2246.
Flott, B., and Siefert, W. 1991. Characterization of glutamate uptake systems in astrocyte primary cultures from rat brain. Glia 4:293–304.
Siesjo, B. K. 1984. Cerebral circulation and metabolism. J. Neurosurg. 60:883–908.
Markov, A. K. 1986. hemodynamics and metabolic effects of fructose 1–6, diphosphate in ischemia and shock-experimental and clinical observations. Annals Emer. Med. 15:1470–1477.
Yudkoff, M., Nissim, I., and Pleasure, D. 1988. Astrocyte metabolism of [15N] glutamine: implications for the glutamine-glutamate cycle. J. Neurochem. 51:843–850.
Kvamme, E., Svenneby, G., Hertz, L., and Schousboe, A. 1982. Properties of phosphate-activated glutaminase in astrocytes cultured from mouse brains. Neurochem. Res. 7:761–770.
Aoki, C., Kaneko, T., Starr, A., and Pickel, V. M. 1991. Identification of mitochondrial and non-mitochondrial glutaminase within select neurons and glia of rat forebrain by electron microscopic immunocytochemistry. J. Neurosci. Res. 28:531–548.
Waniewski, R. A. 1992. Physiological levels of ammonia regulate glutamine synthesis from extracellular glutamate in astrocyte cultures. J. Neurochem. 58(1):167–174.
Benveniste, H., Drejer, J., Schousboe, A., and Diemer, N. H. 1984. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 43:1369–1374.
Sher, P. K., and Hu, S. 1990. Increased glutamate uptake and glutamine synthetase activity in neuronal cell cultures surviving chronic hypoxia. Glia 3:350–357.
Rosenberg, P. A., and Aizenman, E. 1989. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci. Lett. 103:162–168.
Sugiyama, K., Brunori, A., and Mayer, M. L. 1989. Glial uptake of excitatory amino acids influences neuronal survival in cultures of mouse hippocampus. Neurosci. 32:779–791.
Simantov, R. 1989. Glutamate neurotoxicity in culture depends on the presence of glutamine: Implications for the role of glial cells in normal and pathological brain development. J. Neurochem. 52:1694–1699.
Swanson, R. A., Shiraishi, K., Morton, M. T., and Sharp, F. R. 1990. Methionine sulfoximine reduces cortical infarct size in rats after middle cerebral artery occlusion. Stroke 21(2):322–327.
Battaglioli, G., and Martin, D. L. 1991. GABA synthesis in brain slices is dependent on glutamine produced in astrocytes. Neurochem. Res. 16(2):151–156.
Hayashi, M., Hayashi, R., Tanii, H., Hashimoto, K., and Patel, A. J. 1988. The influence of neuronal cells on the development of glutamine synthetase in astrocytes in vitro. Devl. Brain Res. 41:37–42.
Wu, D. K., Scully, S., and De Vellis, J. 1988. Induction of glutamine synthetase in rat astrocytes by cultivation with embryonic chick neurons. J. Neurochem. 50:929–935.
Petito, C. K., Chung, M. C., Verkhovsky, L. M., and Cooper, A. J. L. 1992. Brain glutamine synthetase increases following cerebral ischemia in the rat. Brain Res. 569:275–280.
Richke, R., and Krieglstein, J. 1991. Postischemic neuronal damage causes astroglial activation and increase in local cerebral glucose utilization of rat hippocampus. J. Cereb. Blood Flow Metab. 11:106–113.
Condorelli, D. F., Dell'Albani, P., Kaczmarek, L., Messina, L., Spampinato, G., Avola, R., Messina, A., and Giuffrida Stella, A. M. 1990. Glial fibrillary acidic protein messenger RNA and glutamine synthetase activity after nervous system injury. J. Neurosci. Res. 26:251–257.
Tiffany-Castiglioni, E. C., Peterson, S. L., and Castiglioni, A. J. 1990. Alterations in glutamine synthetase activity by FeCl2-induced focal and kindled amygdaloid seizures. J. Neurosci. Res. 25:223–228.
Tholey, G., Copin, J. C., and Ledig, M. 1991. Hypoxia induced metabolism dysfunction of rat astrocytes in primary cell cultures. Neurochem. Res. 16(4):423–428.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kelleher, J.A., Gregory, G.A. & Chan, P.H. Effect of fructose-1,6-bisphosphate on glutamate uptake and glutamine synthetase activity in hypotix astrocyte cultures. Neurochem Res 19, 209–215 (1994). https://doi.org/10.1007/BF00966818
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00966818