Abstract
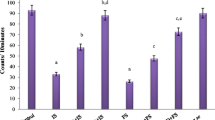
We report earlier that isoniazid and foot-shock stress individually increase the maximal number of [35S]TBPS binding sites (Bmax) measured “ex vivo” in unwashed membranes from rat cerebral cortex and that the increase due to both treatments are prevented by pretreatment “in vivo” with diazepam which alone induced a significant decrease in the total number of [35S]TBPS binding sites. In the present paper, the effect of stress was studied on both the increase in [35S]TBPS binding and the convulsant activity induced by isoniazid in unstressed rats. Isoniazid induced a time dependent increase in [35S]TBPS binding. The isoniazid-induced increase in [35S]TBPS binding was markedly potentiated by foot-shock stress. Moreover, foot-shock stress markedly reduced the latency to the appearance of generalized seizures induced by isoniazid (300 mg/kg s.c.). The results provide evidence that the “in vivo” inhibition of GABAergic transmission elicited by isoniazid results in an increase of [35S]TBPS binding in the rats cerebral cortex. The finding that stress, like isoniazid, enhances [35S]TBPS binding suggests that this treatment also inhibits the function of GABAergic synapses.
Similar content being viewed by others
References
Biggio, G., and Costa, E. 1983. Benzodiazepine Recognition Site Ligands: Biochemistry and Pharmacology. Vol. 38in Advances in Biochemical Psychopharmacology, Raven Press, New York.
Biggio, G., and Costa, E. 1986. GABAergic Transmission and Anxicty. Vol. 41,in Advances in Biochemical Psychopharmacology, Raven Press, New York.
Biggio, G., and Costa, E. 1988. Cl− Channels and their Modulation by Neurotransmitters and Drugs. Vol. 45,in Advances in Biochemical Psychopharmacology, Raven Press, New York.
Biggio, G. 1983. The action of stress, β-carbolines, diazepam and Ro 15-1788 on GABA receptors in the rat brain. Pages 105–117,in Biggio, G., and Costa, E. (eds.), Benzodiazepine Recognition Site Ligands: Biochemistry and Pharmacology, Raven Press, New York.
Biggio, G., Concas, A., Mele, S., and Corda, M. G. 1987. Changes in GABAergic transmission induced by stress, anxiogenic and an xiolytic β-carbolines. Brain Res. Bull. 19:301–308.
Biggio, G., Concas, A., Serra, M., Salis, M., Corda, M. G., Nurchi, V., Crisponi, C., and Gessa, G. L. 1984. Stress and β-carbolines decrease the density of low affinity GABA binding sites: an effect reversed by diazepam. Brain Res. 305:13–18.
Concas, A., Salis, M., Serra, M., Corda, M. G., and Biggio, G. 1983. Ethyl-β-carboline-3-carboxylate decreases3H-GABA binding in membrane preparations of rat cerebral cortex. Eur. J. Pharmacol. 89:179–181.
Concas, A., Serra, M., Atsoggiu, T., and Biggio, G. 1988. Footshock stress and anxiogenic β-carbolines increase35S-TBPS binding in the rat cerebral cortex, an effect opposite to anxiolytics and GABA mimetics, J. Neurochem. 51:1868–1876.
Drugan, R. C., Morrow, A. L., Weizman, R., Weizman, A., Deutsch, S. I. Crawley, J. N., and Paul, S. M. 1989. Stressinduced behavioural depression in the rat is associated with a decrease in GABA receptor-mediated chloride ion flux and brain benzodiazepine receptor occupancy. Brain Res. 487:45–51.
Serra, M., Concas, A., and Biggio, G. 1989. Stress like bicuculline and DMCM reduces the basal35Cl−uptake in the rat cortical membrane vesicles; an effect reversed by GABA. Neurosci. Research Comm. 4:41–50.
Bormann, J. 1988. Electrophysiology of GABAA and GABAB receptor subtypes, TINS 11:112–116.
Braestrup, C., Nielsen, M., Honore, T., Jensen, L. H., and Petersen, E. N. 1983. Benzodiazepine receptor ligands with positive and negative efficacy. Neuropharmacol. 22:1451–1457.
Chan, C. Y., and Farb, D. H. 1985. Modulation of neurotransmission action: control of the γ-aminobutyric acid response through the benzodiazepine receptor. J. Neurosci. 5:2365–2373.
Obata, T., and Yamamura, H. I. 1986. The effect of benzodiazepines and β-carbolines on GABA-stimulated chloride influx by membrane vesicles from the rat cerebral cortex. Bioch. Bioph. Res. Comm. 141:1–6.
Sanna, E., Serra, M., Pepitoni, S., and Biggio, G. 1989. Dramatic increase in nigral [35S]TBPS binding sites elicited by the degeneration of the striato-nigral GABAergic pathway: reversal by diazepam. Brain Res. 501:144–149.
Horton, R. W. 1980. GABA and seizures induced by inhibitors of glutamic acid decarboxylase. Brain Res. Bull. 5:605–608.
Serra, M., Sanna, E., and Biggio, G. 1989. Isoniazid an inhibitor of GABAergic transmission enhances [35S]TBPS binding in rat cerebral cortex. Eur. J. Pharmacol. 164:385–388.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Haefely, W., Kulcsar, A., Mohler, H., Pieri, L., Polc, P., and Schaffner, R. 1975. Possible involvement of GABA in the central actions of benzodiazepines. Vol. 14, Pages 131–151,in Costa E. and Greengard, P. (eds) Advances in Biochemical Psychopharmacology, Raven Press, New York.
Curtis, D. R. 1969, The pharmacology of postsynaptic inhibition. Prog. Brain Res. 31:171–189.
Lothman, E. W., and Collins, R. C. 1981. Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res. 218:299–318.
Ziylan, Y. Z., and Ates, N. 1989. Age-related changes in regional patterns of blood-brain barrier breakdown during epileptiform seizures induced by pentylenetetrazol. Neurosci. Lett. 96:179–184.
Horton, W. R., Chapman, A. G., and Meldrum, B. S. 1979. Isoniazid, as a glutamic acid decarboxylase inhibitor. J. Neurochem. 33:745–750.
Corda, M. G., Costa, E. and Guidotti, A. 1983. Involvement of GABA in the facilitation of punishment-suppressed behaviour induced by β-carbolines in rat. Pages 121–128,in Biggio G., and Costa E. (eds.) Benzodiazepine Recognition Site Ligands: Biochemistry and Pharmacology, Raven Press, New York.
Gee, K. W., Lawrence, L. J., and Yamamura, H. I. 1986. Modulation of the chloride ionophore by benzodiazepine receptor ligands: influence of γ-aminobutyric acid and ligand efficacy. Mol. Pharmacol. 30:218–225.
Squires, R. F., Casida, J. E., Richardson, M., and Saederup, E. 1983. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to γ-aminobutyric acid-A and ion recognition sites. Mol. Pharmacol. 23:326.
Seifert, J., and Casida, J. H. 1985. Regulation of [35S]butylbicyclophosphorothionate binding sites in rat brain by GABA, pyrethroid and barbiturate. Eur. J. Pharmacol. 115:191–198.
Supavilai, P., and Karobath, M. 1984. t-butylbicyclophosphorothionate binding sites are constituents of the γ-aminobutyric acid benzodiazepine receptor complex. J. Neurosci. 4:1193–1200.
Ticku, M. K., and Ramanjaneyulu, R. 1984. Ro 5-4864 inhibits the binding of35S-t-butylbicyclophosphorothionate to rat brain membranes. Life Sci. 34:631–638.
Concas, A., Mele, S., and Biggio, G. 1987. Foot-shock stress decreases chloride efflux from rat brain synaptoneurosomes. Eur. J. Pharmacol. 135:423–427.
Schwartz, R. D., Wess, M. J., Labarca, R., Skolnick, P., and Paul, S. M. 1987. Acute stress enhances the activity of the GABA receptor-gated chloride ionophore ion channel in brain. Brain Res. 411:151–155.
Soubrie, P., Thiebot, M. H., Jobert, A., Montastruc, J. M., Hery, F., and Hamon, M. 1980. Decreased convulsant potency of picrotoxin and pentetrazol and enhanced [3H]flunitrazepam cortical binding following stressful manipulations in rats. Brain Res. 189:505–517.
Trullas, R., Havoundjian, H., and Skolnick, P. 1987. Stress-induced change in t-[35S]butylbicyclophosphorothionate binding to γ-aminobutyric acid-gated chloride channels are mimicked by in vitro occupation of benzodiazepine receptors. J. Neurochem. 49:968–974.
Shivers, B. D., Killisch, I., Sprengel, R., Sontheimer, H., Kohler, M., Schofield, P. R., and Seeburg, P. H. 1989. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 3:327–337.
Squires, R. F., Saederup, E., and Lajtha, A. 1988. Two groups of amino acids interact with GABAA receptors coupled to t-[35S]butylbicyclophorothionate binding sites: possible involvement with seizures associated with hereditary amino acidemias. J. Neurochem. 51:837–842.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Serra, M., Sanna, E., Concas, A. et al. Foot-shock stress enhances the increase of [35S]TBPS binding in the rat cerebral cortex and the convulsions induced by isoniazid. Neurochem Res 16, 17–22 (1991). https://doi.org/10.1007/BF00965822
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00965822