Abstract
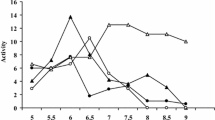
Choline acetyltransferase (ChAT; EC 2.3.1.6) was separated from human caudate/putamen into three fractions by successive extractions into apotassium phosphate buffer, a high salt (NaCl) buffer and a buffer containing 0.6% Triton X-100. The Triton-X-solubilized fraction is the membrane-bound ChAT (mChAT) and represents about 40% of the total ChAT. After centrifugation, mChAT was precipitated by ammonium sulfate at 35–65% saturation. The crude enzyme preparation was fractionated in turn on a DEAE-Sepharose, a hydroxylapatite and a phosphocellulose columns. Finally, mChAT was applied to a CoA-Sepharose column equilibrated with buffer containing 100 mM choline chloride and was specifically eluted with buffer containing acetyl-CoA. The presence of both substrates greatly stabilized the enzyme and ChAT was recovered almost quantitatively. The final preparation of mChAT has a specific activity of 37.2 μmol of acetylcholine synthesized per min-mg protein. The purified mChAT has a pH optimum of 8.3. It migrated as two bands on SDS-PAGE with molecular weights of 67,000 and 62,000 daltons, respectively. Immunoblot autoradiography showed that an antiserum prepared previously against soluble ChAT also cross-reacted with both bands of mChAT, indicating that both forms of this enzyme are related. Furthermore, as previously reported for soluble ChAT, Fab-Sepharose chromatography could be used for the purification of mChAT and this preparation also resolved into two bands of 10% SDS gel.
Similar content being viewed by others
References
Benishin, C. G., andCarroll, P. T. 1981. Differential sensitivity of soluble and membrane-bound forms of choline-O-acetyltransferase to inhibition by coenzyme A. Biochem. Pharmacol. 30:2483–2484.
Benishin, C. G., andCarroll, P. T. 1983. Multiple forms of choline-O-acetyltransferase in mouse and rat brain: Solubilization and characterization. J. Neurochem. 41:1030–1039.
Carroll, P. T., andAspry, J. M. 1980. Subcellular origin of cholinergic transmitter release from mouse brain. Science 210:641–642.
Carroll, P. T., andAspry, J. M. 1981. Spontaneous and potassium induced release of acetylcholine from mouse forebrain minces. Neuroscience 6:2555–2559.
Chibata, L., Tosa, T., andMatuo, K. 1979. Coenzyme A. Methods Enzymol. 34:267–271.
De Robertis, E., Pellegrino de Iraldi, A., Rodriguez de Lores Arnaiz, G., andSalganicoff, L. 1962. Cholinergic and non-cholinergic nerve endings in rat brain I: Isolation and subcellular distribution of acetylcholine and acetylcholinesterase. J. Neurochem. 9:23–35.
De Robertis, E., Rodriguez de Lores Arnaiz, G., andPellegrino De Iraldi, A. 1962. Isolation of synaptic vesicles from nerve endings of the rat brain. Nature 194:794–795.
De Robertis, E., Rodriguez de Lores Arnaiz, G., Salganicoff, L., Pellegrino De Iraldi, A., andZeiher, L. M. 1963. Isolation of synaptic vesicles and structural organization of the acetylcholine system within brain nerve endings. J. Neurochem. 10:225–235.
Dietz, G. W. Jr. andSalvaterra, P. M., 1980. Purification and peptide mapping of rat brain choline acetyltransferase. J. Biol. Chem. 255:10612–10617.
Fonnum, F. 1972. Molecular aspects of compartmentation of choline acetyltransferase. Pages 35–45,in Balázs, R. andCremer, J. E. (eds.). Metabolic Compartmentation in the Brain; Proceedings of a Symposium on Metabolic Compartmentation at the Rockefeller Foundation, Bellagio, Italy. Wiley, NY.
Gray, E. G., andWhittaker, V. P. 1958. The isolation of nerve endings from brain: An electron microscopic study of cell fragments derived by homogenation and centrifugation. J. Anat. Lond. 96:79–88.
Hebb, C. O., andWhittaker, V. P. 1958. Intracellular distribution of acetylcholine and choline acetylase. J. Physiol. 142:187–196.
Hersh, L. B., Wainer, B. H., andAndrews, L. P. 1984. Multiple isoelectric and molecular weight variants of choline acetyltransferase. J. Biol. Chem. 259:1253–1258.
Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., andRandall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Malthe-Sorenssen, D., andFonnum, F. 1972. Multiple forms of choline acetyltransferase in several species demonstrated by isoelectric focusing. Biochem. J. 127:229–236.
McCaman, R. E., Rodriguez De Lores Arnaiz, G., andDe Robertis, E. 1965. Species differences in subcellular distribution of choline acetylase in the CNS. A study of choline acetylase, acetylcholinesterase, 5-hydroxytryptophan decarboxylase, and monoamine oxidase in four species. J. Neurochem. 12:927–935.
McGeer, P. L., McGeer, E. G., andPeng, J. H. 1984. Choline acetyltransferase: Purification and immunohistochemical localization. Life Sci. 34:2319–2338.
Morrissey, J. H. 1981. Silver stain for proteins in polyacrylamide gels: A modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117:307–310.
Peng, J. H., McGeer, P. L., andMcGeer, E. G. 1983. Anti-human choline acetyltransferase fragments antigen binding (Fab)-Sepharose chromatography for enzyme purification. Neurochem. Res. 8:1481–1486.
Peng, J. H., McGeer, P. L., Kimura, H., Sung, S. C., andMcGeer, E. G. 1980. Purification and immunochemical properties of choline acetyltransferase from human brain. Neurochem. Res. 5:943–962.
Peng, J. H., Ma, K., andSung, S. C. 1981. Purification of chicken brain choline acetyltransferase. Neurochem. Int. 3:377–383.
Nelson, S. H., Benishin, C. G., andCarroll, P. T. 1980. Accumulation and metabolism of choline and homocholine by mouse brain subcellular fractions. Biochem. Pharmacol. 29:1949–1957.
Smith, C. P., andCarroll, P. T. 1980. A comparison of solubilized and membranebound forms of choline-O-acetyltransferase (EC 2.3.1.6) in mouse brain, nerve endings. Brain Res. 185:363–371.
Strauss, W. L., andNirenberg, M. 1985. Inhibition of choline acetyltransferase by monoclonal antibodies. J. Neurosci. 5:175–180.
Towbin, H., Staehelin, T., andGordon, J. 1979. Electrophoretic tranfer of proteins from polyacrylamide gels to nitrocellulose sheets; procedure and some applications. Proc. Nat. Acad. Sci. 76:4350–4354.
Author information
Authors and Affiliations
Additional information
Special Issue dedicated to Prof. Eduardo De Robertis.
Rights and permissions
About this article
Cite this article
Peng, J.H., McGeer, P.L. & McGeer, E.G. Membrane-bound choline acetyltransferase from human brain: Purification and properties. Neurochem Res 11, 959–971 (1986). https://doi.org/10.1007/BF00965586
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00965586