Conclusions
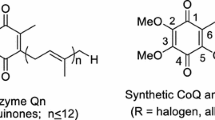
A seven-stage method has been developed for the transformation of ubiquinone-9 to ubiquinone-10 using sulfur-containing synthons as the key step; the most effective synthon was found to be prenyl phenyl sulfide. The analogous homologization of solanesol and decaprenol using an isoprenyl trans-C5-hydroxysulfone was also examined.
Similar content being viewed by others
Literature cited
A. M. Moiseenkov, A. B. Veselovskii, and T. M. Filippova, Izv. Akad. Nauk SSSR, Ser. Khim., 2086 (1987).
A. I. Kozhukhova, E. A. Obol'nikova, and T. M. Filippova, Zh. Org. Khim.,20, 1683 (1984).
S. Terao, M. Shiraishi, K. Kato, et al., J. Chem. Soc., Perkin Trans.,1, 2909 (1982).
K. Sato, O. Miyamoto, S. Inoue, et al., J. Chem. Soc., Chem. Commun., 153 (1982).
M. Julia, D. Uguen, and A. Callipolitis, Bull. Soc. Chim. France, 519 (1976).
K. Shimada, M. Kodama, and S. Ito, Tetrahedron Lett.,22, 4275 (1981).
L. J. Altman, L. Ash, S. Marson, Synthesis, 129 (1974).
Y. Masaki, K. Hashimoto, and K. Kaji, Chem. Pharm. Bull.,32, 3959 (1984).
I. Yoshizawa, H. Toyofuku, K. Tashibana, and T. Kuroda, Chem. Lett., 1131 (1982).
K. Sato, S. Inoue, A. Onishi, N. Uchida, and N. Minowa, J. Chem. Soc., Perkin Trans.,1, 761 (1981).
D. K. Dalling and D. M. Grant, J. Am. Chem. Soc.,94, 5318 (1972).
A. M. Moiseenkov and E. V. Polunin, Izv. Akad. Nauk SSSR, Ser. Khim., 1562 (1983).
C. V. N. Rao and M. K. Chakrpborty, Res. Ind., 24, 83 (1979).
J. Martell and C. Huynh, Bull. Soc Chim. France, 985 (1967).
W. E. Parham and S. H. Groen, J. Org. Chem.,31, 1694 (1966).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 3, pp. 695–701, March, 1988.
Rights and permissions
About this article
Cite this article
Veselovskii, A.B., Moiseenkov, A.M., Filippova, T.M. et al. C5-homologization of ubiquinone-9 and ubiquinone-10 using sulfur-containing synthons. Russ Chem Bull 37, 594–599 (1988). https://doi.org/10.1007/BF00965388
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00965388