Abstract
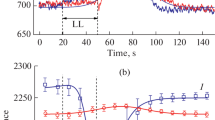
Internal pH (pHi) was determined inEmiliania huxleyi (Lohmann) using the probe 2′,7′-bis-(2-carboxyethyl)-5(and-6)carboxyfluoresceinacetoxymethylester (BCEF-AM) and digital imaging microscopy. The probe BCECF-AM was taken up and hydrolysed to the free acid by the cells. A linear relationship was established between pHi and the 490/450 fluorescence ratio of BCECF-AM over the pH range 6.0 to 8.0 using the ionophore nigericin. Two distinct pH domains were identified within the cell, the cytoplasmic domain (approx. pH 7.0) and the chloroplast domain (approx. pH 8.0). The average pHi was 7.29 (±0.11) for cells in the presence of 2 mM HCO −3 . In the absence of HCO −3 the pHi was decreased by 0.8 pH unit. The importance of these changes in pHi is considered in relation to inorganic-carbon uptake.
Similar content being viewed by others
Abbreviations
- AM:
-
acetoxymethylester
- BCECF:
-
2′,7′-bis-(2-carboxyethyl)-5(and-6)carboxyfluorescein
- Hepes:
-
4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid
- pHi :
-
intracellular pH
References
Beardall, J. (1981) CO2 accumulation byChlorella saccharophila (Chlorophyceae) at low external pH: evidence for active transport of inorganic carbon at the chloroplast envelope. J. Phycol.17, 371–373
Beardall, J., Raven, J.A. (1981) Transport of inorganic carbon and the “CO2 concentrating mechanism” inChlorella emersonii (Chlorophyceae). J. Phycol.17, 134–141
Bright, G.R., Fisher, G.W., Rogowska, J., Lansing-Taylor, D. (1987) Fluorescence ratio imaging microscopy: Temporal and spatial measurements of cytoplasmic pH. J. Cell Biol.104, 1019–1033
Brownlee, C., Pulsford, A. (1989) Visualization of the cytoplasmic Ca2+ gradient inFucus serratus rhizoids: Correlation with cell ultrastructure and polarity. J. Cell. Sci. (in press)
Brownlee, C., Wood, J.W., Briton, D. (1987) Cytoplasmic free calcium in single cells of centric diatoms. The use of Fura-2. Protoplasma.140, 118–122
Burns, B. D., Beardall, J. (1987) Utilization of inorganic carbon by marine microalgae. J. Exp. Mar. Biol. Ecol.107, 75–86
Bush, D.S., Jones, R.L. (1987) Measurement of cytoplasmic calcium in aleurone protoplasts using indo-1 and fura-2. Cell Calcium8, 455–472
Clarkson, D.T., Brownlee, C., Ayling, S.M. (1988) Cytoplasmic calcium measurements in intact higher plant cells: results from fluorescence ratio imaging of fura-2. J. Cell Sci.91, 71–80
Colman, B., Gehl, K.A. (1983) Physiological characteristics of photosynthesis inPorphyridium cruentum: evidence for bicarbonate transport in a unicellular red alga. J. Phycol.19, 216–219
Dixon, G.K., Patel, B.N., Merrett, M.J. (1987) Role of intracellular carbonic anhydrase in inorganic-carbon assimilation byPorphyridium purpureum. Planta172, 508–513
Grynkiewicz, G., Poenie, M., Tsien, R.Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem.260, 3440–3450
Heber, U., Heldt, H.W. (1981) The chloroplast envelope: structure, function and role in leaf metabolism. Annu. Rev. Plant Physiol.32, 139–168
Holligan, P.M., Viollier, M., Harbour, D.S., Camus, P., Champagne-Philippe, M. (1983) Satelite and ship studies of coccolithophore production along a continental shelf edge. Nature304, 339–342
L'Allemain, G., Paris, S., Pouyssegur, J. (1985) Role of a Na+ dependent Cl−/HCO −3 exchange in regulation of intracellular pH in fibroblasts. J. Biol. Chem.260, 4877–4883
McIntyre, A., Bé, A.W.H. (1967) Modern coccolithophorides of the Atlantic Ocean I. Placoliths and cystoliths. Deep-Sea Res.14, 561–597
Olsnes, S., Tonnessen, T.I., Sandvig, K. (1986) pH-regulated anion antiport in nucleated mammalian cells. J. Cell Biol.102, 967–971
Paradiso, A.M., Tsien, R.Y., Machen, T.E. (1987) Digital image processing of intracellular pH in gastric oxyntic and chief cells. Nature325, 447–450
Paasche, E. (1968) Biology and physiology of Coccolithophorids. Annu Rev. Microbiol.22, 71–86
Patel, B.N., Merrett, M.J. (1986) Inorganic-carbon uptake by the marine diatomPhaeodactylum tricornutum. Planta169, 222–227
Pentecost, A. (1985) Calcification and DIC metabolism. In: Inorganic carbon uptake by aquatic photosynthetic organisms, pp. 459–480, Lucas, W.J., Berry, J.A., eds. American Society of Plant Physiologists
Provasoli, L., McLaughlin, J.J.A., Droop, M.R. (1957) The development of artificial media for marine algae. Arch. Mikrobiol.25, 392–428
Raven, J.A. (1980) Nutrient transport in microalgae. Adv. Microb. Physiol.21, 47–226
Raven, J.A., Smith, F.A. (1980) Intracellular pH regulation in the giant-celled marine algaChaetomorpha darwinii. J. Exp. Bot.31, 1357–1369
Rees, T.A.V. (1984) Sodium dependent photosynthetic oxygen evolution in a marine diatom. J. Exp. Bot.35, 332–337
Rink, T.J., Tsien, R.Y., Pozzan, T. (1982) Cytoplasmic pH and free Mg2+ in lymphocytes. J. Cell Biol.95, 189–196
Roos, A., Boron, W.F. (1981) Intracellular pH. Physiol. Rev.61, 296–434
Sikes, C.S., Wilbur, K.M. (1982) Functions of coccolith formation. Limnol. Oceanogr27, 18–26
Sikes, C.S., Roer, R.D., Wilbur, K.M. (1980) Photosynthesis and coccolith formation: Inorganic carbon sources and net inorganic reaction of deposition. Limnol. Oceanogr.25, 248–261
Smith, F.A. (1979) Comparison of the effects of ammonia and methylamine on chloride transport and intracellular pH inChara corallina. J. Exp. Bot.31, 597–606
Thomas, R.C. (1982) Snail neuron intracellular pH regulation. In: Intracellular pH: Its measurement, regulation and utilisation in cellular functions, pp. 189–204, Nuctelli, R., Deamer, D.W., eds. A.R. Liss. Inc., New York
Tromballa, H.W. (1983) The effect of CO2 on potassium transport byChlorella fusca. Plant Cell Environ.6, 537–543
Werdan, K., Heldt, H.W., Mlovanceu, M. (1975) The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim. Biophys. Acta.396, 276–292
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dixon, G.K., Brownlee, C. & Merrett, M.J. Measurement of internal pH in the coccolithophoreEmiliania huxleyi using 2′,7′-bis-(2-carboxyethyl)-5(and-6)carboxyfluorescein acetoxymethylester and digital imaging microscopy. Planta 178, 443–449 (1989). https://doi.org/10.1007/BF00963813
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00963813