Summary
-
1.
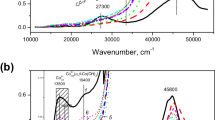
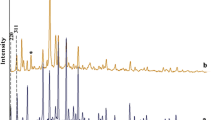
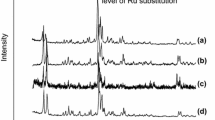
A Ru form of zeolite Y containing the complex [Ru(NH3)4NOOH]2+ has been obtained. Depending on the conditions of the preparative treatments the ruthenium passed into different valence states: Ru2+, Ru+, Ru0.
-
2.
When the complex cations were decomposed in vacuum and were then treated in H2, the Ru clusters were stabilizedpredominantly in the zeoltic cavities. The decomposition of the complex cations in O2 was accompanied by a reduction of the Ru to the metallic state and its migration to the external surface.
-
3.
The reduced forms of Ru zeolites were most active in the NO + CO reaction. The adsorption of NO or O2 at a reaction temperature leading to the oxidation of the Ru and the formation of more oxidized forms of nitrogen as compared with the initial nitrosyl (case of NO) substantially decreased the rate of the reaction.
Similar content being viewed by others
Literature cited
J. H. Lunsford, Adv. Symposium Ser.,40, 39 (1977).
C. Naccache and Y. Ben Taarit, Acta Phys. Chem.,24, 24 (1978).
K. K. Laing, R. Leubner, and J. H. Lunsford, Inorg. Chem.,14, 1400 (1975).
K. C. Taylor and R. L. Kliminish, J. Catal.,30, 478 (1973).
M. L. Unland, Science,179, 567 (1973).
Kh. M. Minachev, G. V. Antoshin, and E. S. Shpiro, Izv. Akad. Nauk SSSR, Ser. Khim., 1015 (1974).
Kh. M. Minachev, G. V. Antoshin, and Yu. A. Yusifov, Adv. Symposium Ser.,40, 559 (1977).
P. H. Citrin, J. Am. Chem. Soc.,95, 6472 (1973).
V. I. Nefedov, Koordinats. Khim.,1, 291 (1975).
J. H. Scofield, J. Electron. Spectr.,8, 129 (1976).
V. I. Nefedov, N. P. Sergushin, I. M. Band, and M. B. Trzhaskovskaya, J. Electron Spectr.,2, 383 (1973).
Kh. M. Minachev. G. V. Antoshin, and E. S. Shpiro, Usp. Khim.,47, 2097 (1978).
J. C. Vedrine, M. Dufaux, C. Naccache, and B. Imelik, J, Chem. Soc. Faraday Trans., No, 1, 440 (1978).
M. Primet, J. C. Vedrine, and C. Naccache, J. Mol. Catal.,4, 411 (1978).
K. S. Kim and N. Winograd, J. Catal.,35, 66 (1974).
O. E. Zvyaginshchev, N. I. Kolbin, A. N. Ryabov, T. D. Avtokratova and A. A. Goryunov, The Chemistry of Ruthenium [in Russian], Nauka (1965).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 6, pp. 1249–1256, June, 1980.
The authors express their gratitude to workers of the Department of X-Ray Diffraetometry of the Hungarian Academy of Sciences (directed by Prof. A. Kalman) for taking the X-ray diffractograms and to Dr. I. Bondar for taking the IR spectra.
Rights and permissions
About this article
Cite this article
Tkachenko, O.P., Shpiro, E.S., Antoshin, G.V. et al. State of ruthenium in a type y zeolite and its catalytic activity in the reduction of no. Russ Chem Bull 29, 873–879 (1980). https://doi.org/10.1007/BF00958798
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00958798