Abstract
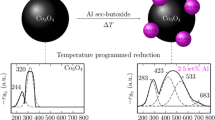
The structure of synthesized cobalt hydroxoaluminate (CHA) and course of thermolysis and reduction were investigated by XPA (including high-temperature x-ray diffraction), thermal chromatography, DTA, DTG, IR, and UV spectroscopy. It was shown that the activity the products of reduction activation of CHA in synthesis of hydrocarbons from CO and H2 is a function of the temperature of reduction and synthesis. It was concluded that hydrocarbons are synthesized on Co/CoAl2O4. The production of CHA are active in the same reduction temperature range as the previously studied cobalt cement catalysts.
Similar content being viewed by others
Literature cited
A. L. Lapidus, I. A. Bruk, V. V. Mal'tsev, et al., Neftekhimiya,22, No. 6, 863 (1981).
A. L. Lapidus, I. A. Bruk, S. D. Sominskii, et al., Neftekhimiya24, No. 1, 45 (1984).
A. L. Lapidus, I. A. Bruk, V. I. Yakerson, et al., Neftekhimiya,20, No. 6 823 (1980).
A. L. Lapidus, I. A. Bruk, V. I. Yakerson, et al., Khim. Tverd. Topl., No. 2, 85 (1984).
Yu. A. Kagan, T. F. Limar', K. A. Shepelenko, and A. S. Mikhailova, Studies at the All-Union Scientific-Research Institute of Single Crystals. Methods of Preparation and Analysis of Materials for Electronics Technology [in Russian], Khar'kov (1976), p. 62.
N. Ya. Turova, Handbook Tables in Inorganic Chemistry [in Russian], Khimiya, Leningrad (1977), p. 92.
C. J. Ballhausen, Introduction to Ligand Field Theory, MgGraw-Hill, New York (1962).
D. T. Sviridov, R. K. Sviridova, and Yu. F. Smirnov, Optical Spectra of Transition Metals in Crystals [in Russian], Nauka, Moscow (1961).
L. H. Little, Infrared Spectra of Adsorbed Species, Academic Press, London (1966).
L. J. Bellamy, The Infrared Spectra of Complex Molecules, 3rd ed., Wiley, New York (1975).
V. D. Nissenbaum, V. I. Yakerson, V. Ya. Danyushevskii, et al., Proceedings of the 9th All-Union Conference on Thermal Analysis [in Russian], Kiev (1985), p. 79.
V. I. Artamonov, E. Z. Golosman, V. I. Yakerson, and A. M. Rubinshtein, Izv. Akad. Nauk SSR, Ser. Khim. No. 5, 988 (1986).
L. A. Pashkevich, V. A. Bronevoi, and I. P. Kraus, Themography of the Products of Alumina Production [in Russian], Moscow (1983).
G. I. Frankfurt, V. I. Yakerson, E. Z. Golosman, and A. L. Lapidus, Proceedings of the All-Union Conference on Chemical Syntheses Based on One-Carbon Molecules [in Russian], Nauka, Moscow (1987), p. 39.
A. L. Lapidus, G. I. Frankfurt, M. D. Sominskii, et al., Use of Optical Spectroscopy in Adsorption and Catalysis. Proceedings [in Russian], Irkutsk (1986), p. 110.
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 4, pp. 759–765, April, 1991.
Rights and permissions
About this article
Cite this article
Lapidus, A.L., Frankfurt, G.I., Yakerson, V.I. et al. Structure of cobalt hydroxoaluminate and catalytic properties of the products of its reduction. Russ Chem Bull 40, 663–668 (1991). https://doi.org/10.1007/BF00958550
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00958550