Conclusions
-
1.
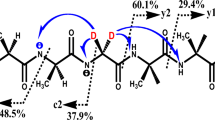
Under chemical ionization conditions, adamantane forms the ions M+. and [M-H]+, while 2,4-dehydroadamantane also gives protonated ([M + H]+) and alkylated ([M + Alk]+) ions.
-
2.
Functional groups in the adamantane molecule undergo ready alkylation and protonation, and may be lost as the HX molecule. Protonation of the diazirine group results in the loss of a molecule of nitrogen. The diazirine group is protonated more readily than the hydroxyl group, but less readily than CN, CONH2, or COOCH3.
Similar content being viewed by others
Literature cited
V. I. Kadentsev, A. V. Krekhin, O. S. Chizhov, and V. V. Ershov, Izv. Akad. Nauk SSSR, Ser. Khim., 1033 (1978).
V. I. Kadentsev, A. Ya. Podel'ko, O. S. Chizhov, et al., Izv. Akad. Nauk SSSR, Ser. Khim., 2015 (1978).
V. I. Kadentsev, A. Ya. Podel'ko, and O. S. Chizhov, Izv. Akad, Nauk SSSR, Ser. Khim., 2686 (1979).
H. Koch and J. Franken, Chem. Ber.,96, 213 (1963).
F. H. Field, in: MTP Internat. Review of Science, Phys. Chem., Series One (A. L. Maccoll, ed., Vol. 5 (1972), p. 138, Butterworths-University Park Press.
V. I. Kadentsev, I. A. Borisova, O. S. Chizhov, et al., Izv. Akad. Nauk SSSR, Ser. Khim., 2457 (1982).
A. A. Solov'ev, V. I. Kadentsev, and O. S. Chizhov, Usp. Khim.,7, 1180 (1979).
F. N. Stepanov and S. S. Guts, Zh. Org. Khim.,6, 1933 (1968).
S. D. Isaev, N. F. Karpenko, A. G. Yurchenko, et al., Proc. 3rd All-Union Conf. on Mass Spectrometry [in Russian], Leningrad (1981), p. 78.
A. C. Udding, J. Strating, and H. Wynberg, Tetrahedron Lett., 1345 (1968).
E. M. Arnett, Current Topics in Physical Organic Chemistry [Russian translation] Mir, Moscow (1967), p. 195.
C. Ford and S. Nagubandi, Tetrahedron,36, 1279 (1980).
L. Vodichka, I. Bukkhard, P. Zakharzh, et al., Zh. Org. Khim.,21, 2569 (1985).
S. D. Isaev, A. G. Yurchenko, Z. N. Murzinova, et al., Zh. Org. Khim.,21, 2669 (1985).
A. G. Yurchenko, S. D. Isaev, and E. F. Novoselov, Zh. Org. Khim.,20, 2227 (1984).
F. N. Stepanov, S. D. Isaev, and Z. P. Vasil'eva, Zh. Org. Khim.,6, 51 (1970).
S. D. Isaev, N. F. Karpenko, G. G. Kolyada, et al., Zh. Org. Khim.,14, 767 (1978).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 9, pp. 2010–2016, September, 1987.
Rights and permissions
About this article
Cite this article
Kadentsev, V.I., Chizhov, O.S., Kolotyrkina, N.G. et al. Behavior of adamantane and its functional derivatives on chemical ionization. Russ Chem Bull 36, 1862–1867 (1987). https://doi.org/10.1007/BF00958331
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00958331