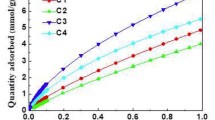
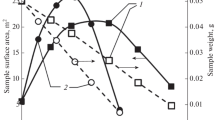
Conclusions
-
1.
A standard adsorption isotherm (CAI) was plotted from the isotherms of adsorption of N2 on nonporous and mesoporous carbon adsorbents for analyzing the pore structure of carbon adsorbents.
-
2.
The analysis of the pore structure of active carbon with a relatively large area of mesopores and predominantly microporous active carbon shows that the CAI obtained adequately describes the adsorption of N2 on the surface of the mesopores of these samples.
Similar content being viewed by others
Literature cited
S. J. Gregg and K. S. Sing, Adsorption, Surface Area, and Porosity, Academic Press, New York (1967).
M. M. Dubinin, Carbon,21, 359 (1983).
M. M. Dubinin, T. I. Izotova, O. Kadlets, and O. L. Krainova, Izv. Akad. Nauk SSSR, Ser. Khim., 1232 (1975).
A. P. Karnaukhov, A. V. Kiselev, and E. V. Khrapova, Dokl. Akad. Nauk SSSR,92, 361 (1953).
S. Ross and W. W. Pultz, J. Colloid Sci.,13, 397 (1958).
N. D. Fedorov, G. K. Ivakhnyuk, and D. N. Gavrilov, Zh. Prikl. Khim.,55, 272 (1982).
A. V. Kiselev and A. A. Isirikyan, Zh. Fiz. Khim.,36, 1164 (1962).
R. Sh. Mikhail, S. Brunauer, and E. E. Bodor, J. Colloid Interface Sci.,26, 45 (1968);26, 54 (1968).
A. Lecloux and J. P. Pirard, J. Colloid Interface Sci.,22, 265 (1979).
G. D. Parfitt, K. S. Sing, and D. Urwin, J. Colloid Interface Sci.,53, 187 (1975).
M. H. Polly, W. D. Schaeffer, and W. R. Smith, J. Phys. Chem.,57, 469 (1953).
C. E. Brown and P. G. Hall, Trans. Faraday Soc.,67, 3558 (1971).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 2, pp. 277–283, February, 1988.
We would like to thank V. F. Surovikin, V. T. Popov, G. A. Andreeva, A. A. Isirikyan, and S. P. Vnukov for making it possible to study samples C-3, CT-1, CT-4-CT-6, CT-8, CT-10, and M-1.
Rights and permissions
About this article
Cite this article
Voloshchuk, A.M., Dubinin, M.M., Moskovskaya, T.A. et al. Pore structure and chemical state of the surface of carbon adsorbents Communication 1. Selection of the comparative isotherm of adsorption of nitrogen vapors on the surface of carbon adsorbents. Russ Chem Bull 37, 204–209 (1988). https://doi.org/10.1007/BF00957410
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00957410