Conclusions
-
1.
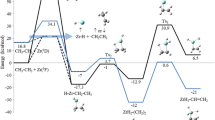
According to MINDO/3 data, the insertion of singlet methylene into the C-H bond of methane and ethane is characterized by an early transition state and by a low activation barrier (∿2 kcal/mole), while the reaction of methylcarbene1CHCH3 with methane is less exothermic and has a later transition state.
-
2.
In the reaction of singlet carbenes with the H2 molecule the successive introduction of alkyl substituents at the carbene center also reduces the exothermic nature of insertion in the series methylene > methylcarbene, ethylcarbene > dimethylcarbene and displaces the transition state toward the final product.
-
3.
According to the calculations and to analysis of the published data, the insertion reactions of singlet carbenes and the reverse processes of carbene fragmentation of alkanes are most sensitive to the introduction of the substituent at the reacting or forming carbene center.
Similar content being viewed by others
Literature cited
R. R. Kostikov, A. F. Khlebnikov, and V. Ya. Bespalov, Zh. Vsesoyuz. Khim., Obshch. im. D. I. Mendeleeva,24, 438 (1979).
M. S. Gordon and D. R. Gano, J. Am. Chem. Soc.,106, 5421 (1984).
C. Soca, J. Am. Chem. Soc.,106, 5847 (1984).
R. C. Bingham, M. J. S. Dewar, and D. H. Lo, J. Am. Chem. Soc.,97, 1285, 1294 (1975).
H. Fisher and H. Kollmar, Theor. Chim. Acta,55, 127 (1980).
E. R. Kyba, J. Am. Chem. Soc.,101, 3217 (1979).
A. I. Ioffe and O. M. Nefedov, Zh. Vsesoyuz. Khim., Obshch. im. D. I. Mendeleeva,24, 475 (1979).
J. B. Pedley and J. Rylance, Computer Analyzed Thermochemical Data: Organic and Organometallic Compounds, Univ. of Sussex (1977).
W. Kirmse, Chemistry of Carbenes [Russian translation], Mir, Moscow (1966).
A. K. Mal'tsev, Zh. Vsesoyuz. Khim., Obshch. im. D. I. Mendeleeva,24, 445 (1979).
J. A. Altmann, I. G. Csizmadia, and K. Yates, J. Am. Chem. Soc.,96, 4196 (1974);97, 5215 (1975).
Yu. V. Tomilov, V. G. Bordakov, A. I. Lutsenko, et al., Izv. Akad. Nauk SSSR, Ser. Khim., 1338 (1987).
A. I. Ioffe, V. A. Svyatkin, and O. M. Nefedov, Izv. Akad. Nauk SSSR, Ser. Khim., 587 (1986).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 5, pp. 1078–1082, May, 1988.
The authors convey their gratitude to V. I. Faustov for providing the MINDO/3 program.
Rights and permissions
About this article
Cite this article
Ioffe, A.I., Nefedov, O.M. Quantum-chemical calculation of the reaction of singlet methylene and its alkyl derivatives with the simplest saturated hydrocarbons and the hydrogen molecule. Russ Chem Bull 37, 942–946 (1988). https://doi.org/10.1007/BF00957066
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00957066