Conclusions
-
1.
TheνP=O absorption band of dimethyl (chloromethyl)-, dimethyl(methoxymethyl)- and dimethyl(phenoxymethyl)phosphine oxides is quite sensitive to complexation with a number of metal salts.
-
2.
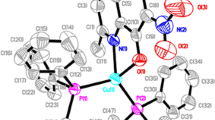
Complexation constants for these phosphine oxides with copper (II) chloride in acetonitrile have been estimated. The stability constants of the [CuClL]+complexes of I–III are close to one another.
Similar content being viewed by others
Literature cited
F. Vogtle and E. Weber, Angew. Chem.,91, 813 (1979).
O. A. Raevskii, M. M. Gilyazov, and Ya. A. Levin, Zh. Obshch. Khim.,48, 1053 (1978).
A. V. Iogansen, Teor. Eksp. Khim.,7, 302 (1971).
M. W. G. De Bolster and W. L. Groenweld, Topics in Phosphorus Chemistry, Vol. 8, Interscience, New York (1976), p. 273.
N. M. Karayannis, C. M. Mikulski, L. S. Gelfand, and L. L. Pytlewsky, J. Inorg. Nucl. Chem.,40, 1513 (1978).
O. A. Raevskii, F. G. Khalitov, A. N. Vereshchagin, and I. M. Vetluzhskikh, Izv. Akad. Nauk SSSR. Ser. Khim., 2446 (1972).
O. A. Raevskii, N. G. Mumzhieva, G. A. Aleksandrova, and R. G. Gainullina, Izv. Akad. Nauk SSSR Ser. Khim., 797 (1977).
I. Ya. Kuramshin, N. R. Safiullina, L. A. Zhelonkina, A. S. Khramov, and A. N. Pudovik, Zh. Obshch, Khim.,48, 2179 (1978).
O. A. Raevskii, N. G. Mumzhieva, T. A. Zyablikova, A. N. Vereshchagin, E. I. Gritsaev, and M. M. Gilyazov, Izv. Akad. Nauk SSSR Ser. Khim., 614 (1978).
É. S. Shcherbakova, I. P. Gol'dshtein, E. N. Gur'yanova, K. A. Kocheshkov, Izv. Akad. Nauk SSSR Ser. Khim., 1262 (1975).
G. E. Forsythe and E. Moler, Computer Solution of Linear Algebraic Systems, Prentice-Hall (1967).
S. E. Manahan and R. T. Iwamoto, Inorg. Chem.,4, 1409 (1965).
Z. A. Sheka, É. I. Sinyavskaya, and M. A. Konstantinovskaya, Ukr. Khim. Zh.,39, 454 (1973).
E. N. Tsvetkov, T. E. Kron, and M. I. Kabachnik, Izv. Akad. Nauk SSSR, Ser. Khim., 669 (1980).
B. M. Gladshtein, V. M. Zimin, V. G. Noskov, L. Z. Soborovskii, B. P. Timoshevskii, and L. N. Shitov, in: Chemistry and Uses of Organophosphorus Compounds. Transactions of the Fourth Conference [in Russian], Nauka, Moscow (1972), p. 279.
H. J. Kleiner, W. Dursch, G. Stahler, and W. Racky, West German Patent 2,258,662 (1974); Ref. Zh. Khim., 10N216P (1975).
A. Gordon and R. Ford, Chemist's Companion: A Handbook of Practical Data, Techniques, and References, Wiley (1973).
G. Schwarzenbach and H. Flaschka, Complexometric Titration [Russian translation], Khimiya, Moscow (1970).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk USSR, Seriya Khimicheskaya, No. 5, pp. 1095–1101, May, 1983.
Rights and permissions
About this article
Cite this article
Raevskii, O.A., Ignat'eva, T.I., Novikov, V.P. et al. Complexing capability of some phosphine oxides containing a dimethylphosphinylmethyl group. Russ Chem Bull 32, 991–995 (1983). https://doi.org/10.1007/BF00956152
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00956152