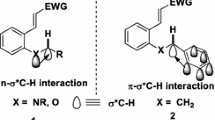
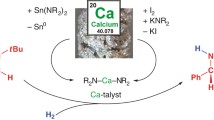
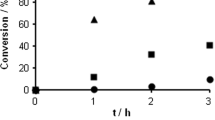
Conclusions
Ethyldichlorosilane and alkylisopropylbenzenes in the presence of aluminum halides are hydride ion donors in the ionic hydrogenation of olefins, alkyl halides, and trifluoroacetate derivatives of alcohols.
Similar content being viewed by others
Literature cited
D. N. Kursanov, Z. N. Parnes, and N. M. Loim, Synthesis, 633 (1974).
D. N. Kursanov, Z. N. Parnes, M. I. Kalinkin, and N. M. Loim, Ionic Hydrogenation [in Russian], Izd. Khimiya, Moscow (1979). p. 85.
V. N. Ipatieff, H. Pines, and R. C. Olberg, J. Am. Chem. Soc.,70, 2123 (1948).
N. Zelinsky, Chem. Ber.,34, 2877 (1901).
M. Mousseron, G. Manon, and G. Combes, Bull. Soc. Chim. Fr., 399 (1949).
V. N. Setkina, D. N. Kursanov, and E. V. Bykova, Izv. Akad. Nauk SSSR, Otd. Khim. Nauk, 1367 (1962).
Huang-Minlon, J. Am. Chem. Soc.,68, 2487 (1946).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 6, pp. 1322–1326, June, 1982.
Rights and permissions
About this article
Cite this article
Bolestova, G.I., Latypova, F.M., Parnes, Z.N. et al. New hydrogenating systems for ionic hydrogenation. Russ Chem Bull 31, 1179–1182 (1982). https://doi.org/10.1007/BF00955973
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00955973