Summary
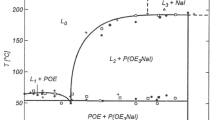
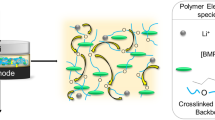
A detailed study was conducted on the dependence of the Tg of polyether networks as a function of type and size of polyether chain, nature of the crosslinking structure and alkali metal salt concentration. The nature of the branching moiety affects Tg but not the salt interactions with the network. A general law concerning the salt-containing network is obtained. It is characterized by the dominating interactions of the salt with polyethylene oxyde chains.
Similar content being viewed by others
References
Brit. Polym. J.20, 171–305 (1988).
J.F. Le Nest, A. Gandini and H. Cheradame, Brit. Polymer. J.20, 253 (1988).
F. Kawakubo, M. Tada, K. Isayama, T. Mita and M. Azuma, Jpn. Kokai Tokkyo Koho32, 597 (1979).
J.F. Le Nest, A. Gandini, H. Cheradame and J.P. Cohen-Addad, Macromolecules21, 1117 (1988).
J.F. Le Nest, A. Gandini, H. Cheradame and J.P. Cohen-Addad, Polym. Commun,28, 302 (1987).
To be presented at the 2nd International Symposium of Polymer Electrolytes, Siena (Italy), June 1989.
Author information
Authors and Affiliations
Additional information
This work has thus allowed the establishment of further general features characterizing the behaviour of polyether networks containing ionic species.
Rights and permissions
About this article
Cite this article
Le Nest, J.F., Gandini, A. Electrolytes for solid-state batteries: glass transition temperature of polyether networks with and without alkali metal salts. Polymer Bulletin 21, 347–351 (1989). https://doi.org/10.1007/BF00955929
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00955929