Conclusions
-
1.
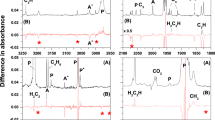
The propargyl radical, obtained by vacuum pyrolysis of propargyl iodide and dipropargly oxalate, has been stabilized in an argon matrix at 12 K and characterized by IR spectroscopy on the basis of 12 of its absorption bands.
-
2.
The vibration frequencies of the propargyl radical have been calculated theoretically, the observed bands have been assigned to normal vibrations, and the force field of this radical has been determined.
-
3.
The values obtained for the stretching vibration frequencies νC≡C, νC-C, νCH, and νCH2 indicate strengthening of the ordinary carbon-carbon bond and weakening of the triple bond in the propargyl radical as a result of electron density delocalization in the conjugated system of orbitals.
Similar content being viewed by others
Literature cited
J. B. Farmer and F. P. Lossing, Can. J. Chem.,33, 86 (1955).
P. H. Kasai, J. Am. Chem. Soc.,94, 5950 (1972); P. J. Krusic and J. K. Kochi, J. Am. Chem. Soc.,92, 4110 (1970); P. J. Krusic and J, K. Kochi, J. Am. Chem. Soc.,90, 7155 (1968); D. H. Volman, K. A. Maas, and J. Wolsteinholme, J. Am. Chem. Soc.,87, 3041 (1965); R. H. Fessenden and R. H. Schuler, J. Chem. Phys.,39, 2147 (1963).
D. A. Ramsay and P. Thistletwaite, Can. J. Phys.,44, 1381 (1966).
J. Collin and F. P. Lossing, Can. J. Chem.,35, 778 (1957).
K. D. King and T. T. Nguyen, J. Phys. Chem.,83, 1940 (1979); K. D. King, Int. J. Chem. Kinet.,2, 23 (1970); W. Tsang, Int. J. Chem. Kinet.,10, 545 (1978).
F. Bernardi, C. M. Camaggi, and M. Tiecco, J. Chem. Soc., Perkin Trans.2, 518 (1974).
H. Honjou, M. Yoshimine, and J. Pacansky, J. Phys. Chem.,91, 4455 (1987).
M. Jacox and D. E. Milligan, Chem. Phys.,4, 45 (1974); L. E. Gusel'nikov, V. V. Volkova, U. Tsigler, et al., Izv. Akad. Nauk SSSR, Ser. Khim., No. 12, 2829 (1986).
J. H. Schachtschneider, Vibrational Analysis of Polyatomic Molecules, Tech. Rep. 231-64, Shell Development Company, Emeryville, CA (1964).
L. M. Sverdlov, M. A. Kovner, and E. P. Krainov, Vibrational Spectra of Polyatomic Molecules [in Russian], Nauka, Moscow (1971).
J. Pacansky and B. Schrader, J. Chem. Phys.,78, 1033 (1983).
M. Spoliti, S. N. Cesaro, and V. Grosso, Spectrochim. Acta A,32, 145 (1976); R. D. Brown, P. J. Domaille, and J. E. Kent, Aust. J. Chem.,23, 1707 (1970).
B. A. W. Coller, M. L. Heffernan, and A. J. Jones, Aust. J. Chem.,21, 1807 (1968).
A. K. Mal'tsev, V. A. Korolev, and O. M. Nefedov, Izv. Akad. Nauk SSSR, Ser. Khim., No. 3, 555 (1984).
J. Pacansky and M. Dupuis, J. Am. Chem. Soc.,104, 415 (1982); J. Pacansky, D. E. Horne, G. P. Gardini, and J. Bargon, J. Phys. Chem.,81, 2149 (1977); J. Pacansky and A. Gutlerres, J. Phys. Chem.,87, 3074 (1983); J. Pacansky, D. W. Brown, and J. S. Chang, J. Phys. Chem.,85, 2562 (1981).
Author information
Authors and Affiliations
Additional information
Deceased.
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 5, pp. 1058–1067, May, 1989.
Rights and permissions
About this article
Cite this article
Korolev, V.A., Mal'tsev, A.K. & Nefedov, O.M. Infrared spectroscopic investigation of propargyl radicals stabilized in low-temperature argon matrix. Russ Chem Bull 38, 957–964 (1989). https://doi.org/10.1007/BF00955425
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00955425