Conclusions
-
1.
Triphenylphosphine complexes of cyanomethylgold and dichlorocyanomethylgold have been synthesized.
-
2.
The chemical properties of aurated acetonitrile were typical for σ organogold compounds but aurated dichloroacetonitrile recalled the chemical behavior of complex gold halides
-
3.
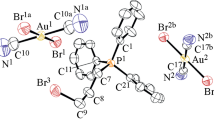
The gold atom in the molecule of dichlorocyanomethyl (triphenylphosphine) gold has linear coordination, the bond lengths and angles were typical for organic complexes of univalent gold.
Similar content being viewed by others
Literature cited
K. I. Grandberg, Usp. Khim.,1, Pt. 3, 438 (1982).
E. I. Smyslova, E. G. Perevalova, V. P. Dyadchenko, K. I. Grandberg, Yu. L. Slovokhotov, and Yu. T. Struchkov, J. Organomet. Chem.,215, 269 (1981).
L. A. Kazitsina, I. F. Lutsenko, G. A. Rudenko, and A. N. Nesmeyanov, Dokl. Akad. Nauk SSSR,127, 115 (1959).
V. P. Dyadchenko, Vestn. Mosk. Unta,17, No. 3, 358 (1976).
L. Lochmann and D. Lim, J. Organomet. Chem.,50, 9 (1973).
R. A. Michelin, M. Napoli, and R. Ros, J. Organomet. Chem.,175, 239 (1979).
H. Schmidbaur and A. Shiotani, Chem. Ber.,104, 2821 (1971).
A. N. Nesmeyanov, E. G. Perevalova, E. I. Smyslova, V. P. Dyadchenko, and K. I. Grandberg, Izv. Akad. Nauk SSSR, Ser. Khim., 2610 (1977).
P. D. Gavens, J. J. Guy, M. J. Mays, and G. M. Sheldrick, Acta Crystallogr.,B33, 137 (1977).
R. W. Baker and P. J. Pauling, J. Chem. Soc. Dalton Trans., 2264 (1972).
L. V. Vilkov, V. S. Mastryukov, and N. I. Sadova, Determination of the Geometric State of Free Molecules [in Russian], Khimiya, Leningrad (1978), p. 118.
G. Matsubayashi, Y. Kondo, T. Tanaka, S. Nishigaki, and K. Nakatsu, Chem. Lett., 375 (1979).
R. G. Gerr, M. Yu. Antipin, N. G. Furmanova, and Yu. T. Struchkov, Krystallografiya,24, 951 (1979).
W. C. Hamilton, Acta Crystallogr.,18, 502 (1965).
E. M. Kaiser and C. R. Hauser, J. Org. Chem.,33, 3402 (1968).
K. I. Grandberg, T. V. Baukova, É. G. Perevalova, and A. N. Nesmeyanov, Dokl. Akad. Nauk SSSR,206, 1355 (1972).
L. H. Jones and R. A. Penneman, J. Chem. Phys.,22, 965 (1954).
A. N. Nesmeyanov, É. G. Perevalova, D. A. Lemenovskii, A. N. Kosina, and K. I. Grandberg, Izv. Akad. Nauk SSSR, Ser. Khim., 2030 (1969).
É. G. Perevalova, D. A. Lemonovskii, K. I. Grandberg, and A. N. Nesmeyanov, Dokl. Akad. Nauk SSSR,202, 93 (1972).
L. H. Jones, J. Chem. Phys.,43, 594 (1965).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 12, pp. 2818–2824, December, 1983.
Rights and permissions
About this article
Cite this article
Perevalova, É.G., Struchkov, Y.T., Dyadchenko, V.P. et al. Cyanomethyl derivatives of gold. Russ Chem Bull 32, 2529–2536 (1983). https://doi.org/10.1007/BF00954488
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00954488