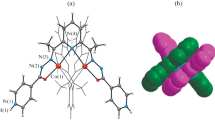
Conclusions
-
1.
During the formation of complexes of Cu(II) with 2-acety1-2,5,5-trimethyl-4-phenyl-3-imidazoline-3-oxide-1-oxyl thiosemicarbazone, which has a donor functional group adjacent to the radical center, in methanol, chloroform, CH2Cl2, or acetone, the nitroxyl group of the ligand is reduced to the hydroxylamine. In alkaline solution, on the other hand, in the presence of Cu(II) salts the hydroxylamine group of 2-acety1-2,5,5-trimethy1-4-phenyl-3-imidazoline-1-hydroxy-3-oxide thiosemicarbazone is converted into the nitroxyl.
-
2.
The coordination compound with the nitroxyl radical is diamagnetic, apparently owing to strong antiferromagnetic interaction between the unpaired electrons of the paramagnetic centers arising as a result of coordination of the
group by the Cu(II) ion.
Similar content being viewed by others
Literature cited
S. V. Larionov, Zh. Strukt. Khim.,23, 125 (1982).
N. V. Kozhemyak, N. V. Podberezskaya, and V. V. Bakakin, Zh. Strukt. Khim.,21, 124 (1980).
J. F. W. Keana, J. Cuomo, L. Lex, and S. E. Seyedrezai, J. Org. Chem.,48, 2647 (1983).
Yu. G. Mamedova, A. A. Medzhidov, and L. N. Kolomina, Zh. Neorg. Khim.,17, 2946 (1972).
A. A. Medzhidov, T. M. Kutovaya, N. P. Rodin, M. K. Guseinova, I. A. Timakov, and Kh. S. Mamedov, Koordinats, Khim.,5, 1433 (1979).
N. P. Rodin, A. A. Medzhidov, T. M. Kutovaya, and K. K. Guseinova, Zh. Strukt. Khim.,22, 85 (1981).
I. A. Grigor'ev, G. I. Shchukin, S. A. Dikanov, I. K. Kuznetsov, and L. B. Volodarskii, Izv. Akad. Nauk SSSR, Ser. Khim., 1092 (1982).
V. M. Shul'man, Z. A. Savel'eva,. I. M. Cheremisina, Ya. V. Vasil'ev, and Ya. V. Anufrieitko, Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, No. 1, 77 (1972); V. M, Shul'man, V. A. Savel'eva, and R. I. Novoselova, ibid., No. 2, 55 (1972).
V. G. Bodyu and N. V. Gérbéléu, Zh. Neorg. Khim.,17, 2159 (1972).
D. Jahr, K. E. Schwarzhans, D. Nöthe, and P. Burkert, Z. Naturforsch.,B26b, 1210 (1971).
W. J. Geary, Coord. Chem. Rev.,7, 81 (1971).
W. Brackman and C. J. Gaasbeek, Rec. Trav. Chim.,85, 221 (1966).
G. A. Bagyan, A. K. Valeev, I. K. Loroleva, and N. V. Soroka, Zh. Neorg. Khim.,28, 2016 (1983).
I. V. Berezin and A. A. Klesov, A Practical Course in Chemical and Enzymic Kinetics [in Russian], Izd. Moskovsk. Univ., Moscow (1976), pp. 20–22.
V. I. Ovcharenko, S. V. Larionov, V. K. Mokhosoeva, and L. B. Volodarskii, Zh. Neorg. Khim.,28, 151 (1983).
V. D. Sen, V. A. Golubev, and A. N. Rosenberg, Symposium on Stable Nitroxyl Radicals, Pecs. Hungary (1979), pp. 38–39.
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 1, pp. 162–168, January, 1986.
Rights and permissions
About this article
Cite this article
Ovcharenko, V.I., Sorokina, T.N., Ikorskii, V.N. et al. Coordination compounds of Cu(II) with the nitroxyl radical 2-acetyl-2,5,5-trimethyl-4-phenyl-3-imidazoline-3-oxide-1-oxyl thiosemicarbazone and its 1-hydroxy derivative. Russ Chem Bull 35, 148–153 (1986). https://doi.org/10.1007/BF00952863
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00952863