Conclusions
-
1.
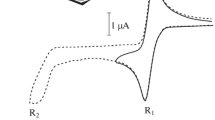
The possibility of electrochemical generation and identification of metastable molybdocene and tungstocene, formed as a result of irreversible two-electron reduction of Cp2MoCl2 and Cp2WCl2, respectively, on a mercury electrode, was demonstrated. The values of the redox potentials of the couples Cp2Mo0/+ and Cp2W0/+ were determined.
-
2.
In contrast to stepwise two-electron reduction of Cp2TiCl2 and Cp2VCl2, the reduction of Cp2MoCl2 and Cp2WCl2 proceeds virtually in one two-electron step, which is due to the 18-electron configuration of the initial complexes of metals of subgroup VIB.
-
3.
Electroreduction of the complexes Cp2MCl2 (M=Mo and W) in an atmosphere of CO leads to the formation of more stable carbonyl complexes Cp2M(CO) in comparison with the corresponding metallocenes. Coordination with CO most probably occurs at the stage of products of one-electron reduction of Cp2MCl2.
Similar content being viewed by others
Literature cited
A. N. Nesmeyanov and K. A. Kochetkov (eds.), Methods of Heteroorganic Chemistry. The Copper, Scandium, Titanium, Vanadium, Chromium, and Manganese Subgroups. The Lanthanides and Actinides [in Russian], Vol. 1, Nauka, Moscow (1974).
J. L. Thomas and H. H. Brintzinger, J. Am. Chem. Soc.,94, 1386 (1972).
K. L. Tang Wong, J. L. Thomas, and H. H. Brintzinger, J. Am. Chem. Soc.,96, 3694 (1974).
P. Grebenik, A. J. Downs, M. L. Green, and R. N. Perutz, J. Chem. Soc. Chem. Communs, 742 (1979).
J. Chetwynd-Talbot, P. Grebenik, and R. N. Perutz, Inorg. Chem.,21, 3647 (1982).
V. V. Strelets, V. N. Tsarev, and O. N. Efimov, Dokl. Akad. Nauk SSSR,259, 646 (1981).
V. V. Strelets and V. N. Tsarev, Kinet. Katal.,24, 73 (1983).
M. L. H. Green and W. C. Lindsell, J. Chem. Soc., 1155 (1967A).
V. V. Strelets, G. L. Soloveichuk, A. I. Sizov, B. M. Bulychev, A. Rusina, and A. A. Vlchek, Izv. Akad. Nauk SSSR, Ser. Khim., 2493 (1983).
J. C. Kotz, W. Vining, W. Coco, R. Rosen, R. Dias, and M. H. Garcia, Organometallics,2, 68 (1983).
R. S. Nicholson and I. Shain, Anal. Chem.,36, 706 (1964).
J. D. L. Holloway and W. E. Geiger, J. Am. Chem. Soc.,101, 2038 (1979).
Y. Mugnier, C. Moise, J. Tirouflet, and E. Laviron, J. Organomet. Chem.,186, C49 (1980).
Y. Mungier, C. Moise, and E. Laviron, Nouv. J. Chim.,6, 197 (1982).
V. V. Strelets and S. V. Kukharenko, Dokl. Akad. Nauk SSSR,275, 894 (1984).
N. El Murr, C. Moise, M. Riveccie, and J. Tirouflet, Inorg. Chim. Acta,32, 189 (1979).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 7, pp. 1484–1490, July, 1985.
Rights and permissions
About this article
Cite this article
Strelets, V.V., Kuzharenko, S.V., Soloveichuk, G.L. et al. Electrochemistry of molybdocene dichloride and tungstocene dichloride and the identification of metastable metallocenes. Russ Chem Bull 34, 1357–1362 (1985). https://doi.org/10.1007/BF00950129
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00950129