Conclusions
-
1.
A study has been made of the reaction of methyl viologen by the ketyl radical in micellar ionic surfactants.
-
2.
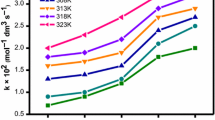
The reaction rate constant is subject to the equation of the second approximation of the Debye-Hückel theory when the process is carried out in micellar systems.
Similar content being viewed by others

Literature cited
R. Hilhorst, C. Laane, and C. Veeger, Proc. Nat. Acad. Sci. USA,79, 3927 (1982).
K. Shinoda, T. Nakagawa, B. Tamamusi, and T. Isemura, Colloidal Surfactants, Academic Press, New York (1963).
K. L. Mittal (ed.), Micellization, Solubilization, and Microemulsions, Plenum Press, New York (1977).
D.-J. Lougnot, R. Jacques, and J.-P. Fouassier, J. Photochem.,19, 59 (1982).
K. J. Mysels and L. H. Princen, J. Phys. Chem.,63, 1696 (1959).
N. A. Mazer and G. Olofson, J. Phys. Chem.,86, 4584 (1982).
M. Almgren and S. Swarup, J. Phys. Chem.,86, 4212 (1982).
G. N. Kryukova, V. A. Kasaikin, Z. N. Markina, and A. V. Sineeva, Kolloidn. Zh.,53, 660 (1981).
M. B. Ledger and G. Porter, J. Chem. Soc., Faraday Trans, 1,68, 539 (1972).
R. V. Bensasson and J.-C. Gramain, J. Chem. Soc., Faraday Trans, 1,76, 1801 (1980).
A. M. Braun, M. Krieg, N. J. Turro, M. Aikawa, I. R. Gould, G. A. Graf, and P. C.-C. Lee, J. Am. Chem. Soc.,103, 7312 (1981).
P. S. Rao and E. Hayon, J. Am. Chem. Soc.,96, 1287 (1974).
J. C. Scaiano, E. B. Abuin, and L. C. Stewart, J. Am. Chem. Soc.,104, 5673 (1982).
J. H. Fendler, E. J. Fendler, G. A. Infante, P.-S. Shih, and L. K. Patterson, J. Am. Chem. Soc.,97, 89 (1975).
R. Breslow, S. Kitabatake, and J. Rothbard, J. Am. Chem, Soc.,100, 8156 (1978).
D. Bendedouch, Hsin Chen Sow, and W. C. Koehler, J. Phys. Chem.,87, 153 (1983).
I. V. Berezin, K. Martinek, and A. K. Yatsimirskii, Usp. Khim,42, 1729 (1973).
M. S. Fernandez, and P. Fromherz, J. Phys. Chem.,81, 1755 (1977).
C. A. Bunton and L. Sepulveda, J. Phys. Chem.,83, 680 (1979).
C. A. Bunton and L. Robinson, J. Am. Chem. Soc.,90, 5972 (1978).
P. Debye, Ann. N. Y. Acad. Sci.,51, 575 (1949).
M. Grätzel and J. K. Thomas, J. Phys. Chem.,78, 2248 (1974).
D. Meisel, M. S. Matheson, and J. Rabant, J. Am. Chem. Soc.,100, 117 (1978).
Y. Moroi, A. Braun, and M. Grätzel, J. Am. Chem. Soc.,101, 567 (1979).
P.-A. Brugger, P. P. Infelta, and M. Grätzel, J. Am. Chem, Soc.,103, 320 (1981).
G. V. Fomin, M. M. Shabarchina, and Yu. Sh. Moshkovskii, Zh. Fiz. Khim.,53, 2099, 2101, 2358, 2360 (1979);54, 2400 (1980).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 11, pp. 2462–2468, November, 1984.
The authors wish to thank A. E. Shilov, Corresponding Member of the Academy of Sciences of the USSR, for valuable advice on this work.
Rights and permissions
About this article
Cite this article
Burbo, E.M., Gasanova, L.V. & Dzhabiev, T.S. Redox reactions in micellar systems Communication 2. Influence of salts on rate of reduction of methyl viologen. Russ Chem Bull 33, 2252–2257 (1984). https://doi.org/10.1007/BF00948832
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00948832