Conclusions
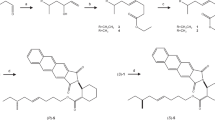
An effective four-stage synthetis of (±)-frontalin from mesityl oxide has been developed with a low-temperature oxy-Cope rearrangement as the key stage.
Similar content being viewed by others
Literature cited
W. D. Bedard and D. L. Wood, in: Management of Insect Pests with Semichemicals: Concepts and Practice, E. R. Mitchell (ed.), Plenum Press, New York (1981); T. L. Payne, in: Management of Insect Pests with Semichemicals: Concepts and Practice, E. R. Mitchell (ed.), Plenum Press, New York (1981), p. 103.
G. W. Kintzer, A. F. Fentman, T. F. Page, R. L. Foltz, J. P. Vite, and G. B. Pitman, Nature,221, 477 (1969).
B. P. Mundy, R. D. Otzenberger, and A. R. DeBernardis, J. Org. Chem.,36, 2390 (1971).
T. D. J. D'Silva and D. W. Peck, J. Org. Chem.,37, 1838 (1972).
K. Mori, S. Kobayashi, and M. Matsui, Agr. Biol. Chem.,43, 1889 (1975).
K. von Fraunberg, BRD Offenlegungschrift 2341840 (1975); Chem. Abstr.,82, P170874 (1975).
P. E. Sum and L. Weiler, Can. J. Chem.,57, 1475 (1979).
R. M. Wilson and J. W. Rekers, J. Am. Chem. Soc.,103, 206 (1981).
C. Meister and H. D. Sharf, Liebigs Ann. Chem., B, 913 (1983).
V. S. Markevich, L. V. Andreev, and E. O. Borisova, Inventor's Certificate No. 230123 (1968) (USSR); Byull. Izobret., No. 34 (1969).
I. Fujita, T. Ohnishi, and T. Nisida, Nippon Kokkai Tokkoe Kocho 52-73726. Japanese Patent No. 79-73726 (1979); Chem. Abstr.,91, P 210911 (1979).
G. F. Woods and A. Viola, J. Am. Chem. Soc.,78, 4380 (1956).
A. Viola, E. J. Iorio, K. K. Cohen, G. M. Glover, U. Nayak, and P. Kocienski, J. Am. Chem. Soc.,89, 3462 (1967).
Y. Fujita, T. Ohnishi, and T. Nishida, Synthesis, 934 (1978).
L. Overman and F. M. Knoll, J. Am. Chem. Soc.,103, 865 (1980).
L. D. Melikadze, L. D. Mikadze, D. Sh. Shonya, Z. I. Gurgenidze, Sh. Sh. Barbanadze, and I. I. Abkhazava, Soobshch. Akad. Nauk Gruz. SSR, No. 65(2) 474 (1972).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 8, pp. 1890–1892, August, 1985.
Rights and permissions
About this article
Cite this article
Serebryakov, É.P., Gamalevich, G.D. Attractant pheromones of coleoptera. Communication 2. Simple synthesis of (±)-frontalin using low-temperature oxy-cope rearrangement. Russ Chem Bull 34, 1740–1742 (1985). https://doi.org/10.1007/BF00948529
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00948529