Abstract
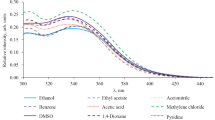
A number of aryloxazol-5-ones were synthesized by condensation of aromatic aldehydes with hippuric acid and substituted hippuric acids. Most of the products have intense luminescence in the solid state; all of them have luminescence in toluene at 77°K. Lengthening of the conjugation chain in the oxazolone molecules leads to a shift in their luminescence to the long-wave region. The compound that contains a conjugated system including two oxazolone rings also luminesces intensely in toluene at room temperature.
Similar content being viewed by others
Literature cited
I. Plöchl, Ber.,16, 2815 (1883).
E. Erlenmeyer, Ann.,275, 1, (1893).
B. M. Krasovitskii and B. M. Bolotin, Organic Luminophores [in Russian], Khimiya, Leningrad (1976).
D. Bassi, D. Deulofeu, and F. Ortega, J. Am. Chem. Soc.,75, 171 (1953).
E. L. Bennett and E. Hoerger, J. Am. Chem. Soc.,74, 5975 (1952).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 158–160, February, 1978.
Rights and permissions
About this article
Cite this article
Krasovitskii, B.M., Lysova, I.V. & Afanasiadi, L.S. Synthesis and spectral-luminescence properties of 2-aryloxazol-5-ones. Chem Heterocycl Compd 14, 120–122 (1978). https://doi.org/10.1007/BF00945320
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00945320