Abstract
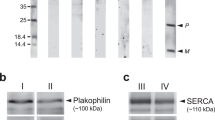
The purpose of the present study was to compare protein profiling of atria and ventricles in children operated for congenital heart disease. Tissue samples were obtained during surgery from patients with normoxemic (ventricular and atrial septal defects) and hypoxemic (tetralogy of Fallot) diseases. Protein fractions were isolated by stepwise extraction from both fight ventricular and atrial musculature. The concentration of total atrial protein in the normoxemic patients exceeded the ventricular value (110±2.1 vs 99.9±4.0mg.g−1 wet weight, respectively); in the hypoxemic group this atrio-ventricular difference disappeared. The concentration of contractile proteins in all cardiac samples was significantly higher in the ventricles as compared with atria, while the concentration of collagenous proteins was significantly higher in the atria (due to a higher amount of the insoluble collagenous fraction). The concentration of sarcoplasmic proteins (containing predominantly enzyme systems for aerobic and anaerobic substrate utilization), however did not differ between ventricles and atria. Furthermore, ventricular contractile fractions obtained from both normoxemic and hypoxemic patients were contaminated with the myosin light chain of atrial origin. Soluble collagenous fractions (containing newly synthesized collagenous proteins, predominantly collagen I and III), derived from all ventricular samples, were contaminated by low molecular weight fragments (mol. weight 29–35 kDa). The proportion of the soluble collagenous fraction was significantly higher in atrial but not in ventricular myocardium of hypoxemic children as compared with the normoxemic group. It seems, therefore, that lower oxygen saturation affects the svnthesis of collagen preferentially in atrial tissue.
Similar content being viewed by others
References
Gorza L, Sartore S, Schiaffino S: Myosin types and fiber types in cardiac muscle. II-Atrial myocardium. J Cell Biol 95: 838–845, 1982
Sartore S, Gorza L, Bormioli SP, Dalla-Libera L, Sciaffino S: Myosin types and fiber types in cardiac muscle. I-Ventricular myocardium. J Cell Biol 88: 226–233, 1981
Sommer JR, Jennings RB: Ultrastructure of cardiac muscle in: Fozzard et al. (eds). The heart and cardiovascular system. Raven Press Ltd. New York, 1992, pp 3–50
Arminger LC, Seelye RN, Morrison MA, Holliss DG: Comparative biochemistry and fine structure of atrial and ventricular myocardium during autolysisin vitro. Basic Res Cardiol 79: 218–229, 1984
Dalla-Libera L, Carraro U, Pauletto P: Light and heavy chains of myosin from atrial and ventricular myocardium of turkey and rat. Basic Res Cardiol 78: 671–678, 1983
Dechesne C, Legher J, Bouvagnet P, Claviez M, Leger JJ: Fractionation and characterization of two molecular variants of myosin from adult human atrium. J Mol Cardiol 17: 753–767, 1985
Swynghedauw B: Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev 66: 710–711, 1986
Weber K: Cardiac interstitium: Extracellular space of the myocardium. In: H.A. Fozzard et al. (eds). The heart and Cardiovascular system Raven press Ltd., New York, 1992, pp 1465–1480
Borg TK, Terracio L. Interaction of the extracellular matrix with cardiac myocytes during development and disease. In: T.F. Robinson, R.K.H. Kinne, (eds). Cardiac myocyte-connective tissue interactions, in health and disease Basel: 13, 1990, pp 113–129
Borg TK, Burgess ML: Holding it all together: Organization and function of the extracellular matrix in the heart. Heart Failure 8 230–238, 1992
Pelouch V, Jirmar R: Biochemical characterization of cardiac collagen and its role in ventricular remodelling following infarction. Physiol Res 42: 283–292, 1993
Pelouch V, Dixon IMC, Golfronan L, Beamish RE, Dhalla NS: Role of extracellular matrix proteins in heart function. Mol Cell Biochem 129: 101–120, 1994
Caufield JB, Norton P, Weaver D: Cardiac dilatation associated with collagen. Mol Cell Biochem 118: 171–179, 1992
Weber K: Cardiac interstitium in health and disease. The fibrilar collagen network. J Am Coll Cardiol 13: 1637–1652, 1989
Imataka K, Naito S, Seko Y, Fujii J: Hydroxyproline in all part of the rabbit heart in hypertension and in its reversal. J Mol Cell Cardiol 21: (Suppl. V) 133–139, 1989
Pelouch V, Milerova M, Ostadal B, Samanek M, Hucin B: Protein profiling of human atrial and ventricular musculature: the effect of normoxemia and hypoxemia in congenital heart diseases. Physiol Res 42: 235–242, 1993
Bass A, Šamánek M, Ošt'ádal B, Hucin B, Stejskalová M, Pelouch V: Differences between atrial and ventricular energy supplying enzymes in children. J App Cardiol 3: 397–405, 1988
Pelouch V, Milerová M, Šamánek M, Ošt'ádal B, Hučin B: Protein composition of the atria and ventricles in children with congenital heart disease. Physiol bohemoslov 37: 530–531, 1988
Yazaki Y, Tsuchimochi H, Kurabayashi M, Komuro I: Molecular adaptation to pressure overload, in human and rat hearts. J Mol Cell Cardiol 21: 91–101, 1989
Pelouch V, Oštádal B, Kolář F, Milerová M, Grünermel J: Chronia hypoxia-induced age-dependent changes of collagenous and noncollagenous cardiac protein fractions. In: B. Oštádal, N.S. Dhalla (eds). Heart Function in Health and Disease. Kluwer Academic Publishers, Boston/Dordrecht/London, 1992, pp 209–218
Pelouch V, Dixon IMC, Sethi R, Dhalla NS: Alteration of collagenous protein profile in congestive heart failure secondary to myocardial infarction. Mol Cell Biochem 129: 121–131, 1994
Lowry OH, Rosenbrough HJ, Farr AL, Randall RJ: Protein measurement with Folin phenol reagent. J Biol Chem 193: 265–275, 1951
Huszar G: Monitoring of collagen and collagen fragments in chromatography of protein mixture. Anal Biochem 105: 424–429, 1980
Lockard VG, Bloom S: Trans-cellular desmin-laminin B intermediate filament network in cardiac myocytes. J Mol Cell Cardiol 25: 303–309, 1993
Samánek M, Bass A, Ošt'ádal B, Hucin B, Stejskalová M: Effect of hypoxaemia on enzymes supplying myocardial energy in children with congenital heart disease. Int J Cardiol 25: 265–270, 1989
Humprey JE, Cummins P: Regulatory proteins of the myocardium. Atrial and ventricular tropomyosin and troponin in the developing and adult bovine and human heart. J Mol Cell Cardiol 16: 647–657, 1984
Nakanishi T, Okuda H, Kamata K, Abe K, Sekiguchi M, Takao A: Development of myocardial contractile system in fetal rabbit. Pediatric Res 22: 201–207, 1987
Malhotra A, Siri FA, Aronson R: Cardiac contractile proteins in hypertrophied and failing guinea pig heart. Cardiovasc Res 26: 153–162, 1992
Dhalla NS, Dixon IMC, Beamish RE: Biochemical basis of heart function and contractile failure. J Appl Cardiol 6: 7–30, 1991
Hirzel HO, Tuchschmid CR, Schneider J, Krayenbuehl HP, Schaub MC: Relationship betwen myosin isoenzyme composition, hemodynamics and myocardial structure in various forms of human cardiac hypertrophy. Circ Res 57: 729–740, 1985
Hoffman U, Siegert E: Atrial and ventricular myosins from human hearts: I): Isoenzyme distribution during development and in the adults. Basic Res Cardiol 82: 348–358, 1987
Hoffman U, Axmann C, Palm N: Atrial and ventricular myosins from human hearts. II): Isoenzyme distribution after myocardial infarction. Basic Res Cardiol 82: 359–369, 1987
Eghbali M, Czaja MJ, Zeydel M, Weiner FR, Seifter S, Blumenfeld OO: Collagen chain mRNAs in isolated heart cells from young and adult rats. J Mol Cell Cardiol 20: 267–276, 1988
Kawahara E, Mukai A, Oda Y, Nakanishi I, Iwa T: Left ventriculotomy of the heart: tissue repair and localization of collagen types I, II, III, IV, V, VI and fibronectin. Virchows Archiv 417A: 229–236, 1990
Caspari PG, Gibbson K, Harris P: Changes in myocardial collagen in normal development and after beta blockade. In: Recent advances in studies on cardiac structure and metabolism. Academic Press New York, vol. 7, 1976, pp99–104
Kardami E, Fandrich RR: Basic fibroblast growth factor in atria and ventricles of the vertebrate heart. J Cell Biol 109: 1865–1874, 1989
Pelouch V, Ošt'ádal B, Procházka J, Urbanová D, Widimský J: Effect of high altitude hypoxia on the protein composition of the right ventricular myocardium. Prog Resp Res 20: 41–48, 1985
Pelouch V, Ošt'ádal B, Procházka J: Changes of contractile and collagenous protein induced by chronic hypoxia in myocardium during postnatal development of rat. Biomed Biochim Acta 46: S707-S711, 1987
Hollenber M, Honbo N, Samorodin AJ: The effect of hypoxia on cardiac growth in neonatal rat. Am J Physiol 231: 1445–1450, 1976
Holenber M, Honbo N, Samorodin AJ: Cardiac cellular responses to altered nutrition in neonatal rat. Am J Physiol 233: H356-H360, 1977
Laemmli UK: Cleavage of structural proteins during the assembly of the heaqd of bacteriophafe T4. Nature 227: 680–685, 1970
Factor SM: Pathological alteration of myocyte-connective tissue interaction in cardiovascular disease. In: T.F. Robinson, R.K.H. Kinne (eds). Cardiac myocyte-connective tissue interactions, in health and disease. Basel: 13, 1990, pp 130–146
Laurent GJ: Dynamic state of collagen: pathways of collagen degradationin vivo and their possible role in regulation of collagen mass. Am J Physiol 252: C1-C9, 1987
Schapper J, Froede R, Hein S, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N: Impairment of the myocardial ultrastructure and changes of cytoskeleton in dilated cardiomyopathy. Circulation 83: 504–514, 1991
Weber KT, Janicki JS, Pick R, Capasso J, Anversa P: Myocardial fibrosis and pathological hypertrophy in the rat with renovascular hypertension. Am J Cardiol 65: G1-G7, 1990
Kivirikko KI: Collagens and their abnormalities in wide spectrum of diseases. Ann Med 2: 113–126, 1993
Price KM, Littler WA, Cummins P: Human atrial and ventricular myosin light chain subunits in the adult and during development. Biochem J 191: 571–580, 1980
Cummins P: Transitions in human atrial and ventricular myosin light chain isoenzymes in response to cardiac pressure overload-induced hypertrophy. Biochem J 205: 195–204, 1982
Schaub MC, Hirzel HO: Atrial and ventricular isomyosin composition in patients with different forms of cardiac hypertrophy. Basic Res Cardiol 82: (Suppl. 2) 357–367, 1987
Nakao K, Yasue H, Fujimoto K, Okumura K, Yamoto H, Hitoshi Y, Murohara T, Takatsu K, Miyamoto E: Increased expression of atrial myosin light chain 1 in the overloaded human left ventricle: possible expression of fetal type myocytes. Inter J Cardiol 36: 315–328, 1992
Trahair T, Yeoh T, Cartmill T, Keogh A, Spratt P, Chang V, dos Remedios CG, Gunning P: Myosin light chain gene expression associated with disease states of the human heart. J Mol Cell Cardiol 26: 577–585, 1993
Takeda N, Rupp H, Fenchel G, Hofmeister H-E, Jacob R: Relationship between the myofibrillar ATPase activity of human biopsy material and hemodynamic parameters. Jpn Heart J 26: 909–922, 1985
Bass A, Stejskalová M, Šamánek M, Ošt'ádal B, Hučin J, Procházka J: Comparison of energy-supply enzyme activities in the right atrium and ventricle of children with congenital heart disease. Physiol bohemoslov 37: 530–531, 1988
Morgan HF, Baker KM: Cardiac hypertrophy. Mechanical, neural and endocrine dependence. Circulation 83: 13–25, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pelouch, V., Milerová, M., Ošťádal, B. et al. Differences between atrial and ventricular protein profiling in children with congenital heart disease. Mol Cell Biochem 147, 43–49 (1995). https://doi.org/10.1007/BF00944782
Issue Date:
DOI: https://doi.org/10.1007/BF00944782