Abstract
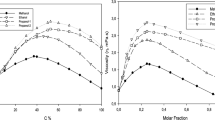
It has been shown that part of the free volume of a solvent attaching to the solvation shell must be excluded from the total free volume in the Bachinskii equation. This determines the increase of viscosities of solutions with positive solvation. An equation has been obtained for the inverse relative viscosity of solutions η0/η = 1-zN2/(1-N2), where no and η are the dynamic viscosities respectively of the solvent and the solution; z is the solvation number, N2 is the mole fraction of dissolved material. A method is proposed for determining the solvation number (hydration) from solution viscosity data. Solvation numbers obtained by this method are in good agreement with values of z obtained from the literature and determined by other methods.
Similar content being viewed by others
Literature cited
G. N. Panchenkov, Theory of Viscosity of Liquids [in Russian], Gostoptekhizdat, Moscow (1947).
Ya. I. Frenkel, Kinetic Theory of Liquids [in Russian], Nauka, Leningrad (1975).
T. Erdy-Gruz, Transport Phenomena in Aqueous Solutions, Halsted Press (1974).
R. Robinson and R. Stokes, Electrolyte Solutions, Academic Press (1959).
L. L. Ezrokhi, “Viscosity of solutions of the systems Na+-K+/Cl−-SO 2−4 /H2O,” Tr. VNII Galurg., No. 27, 113–131 (1953).
A. M. Sukhotin (editor), Handbook of Electrochemistry [in Russian], Khimiya, Leningrad (1981).
D. Feakins and K. G. Lawrence, “The relative viscosities of solutions of sodium and potassium chlorides and bromides in N-methylformamide at 25, 35 and 45°C,” J. Chem. Soc. A, No. 2, 212–219 (1966).
T. V. Rebagay, J. F. Casteel, and P. G. Sears, “Conductance-viscosity studies on some moderately concentrated nonaqueous electrolyte solutions from -50 to 125°C. 1. Solutions of Bu4NI, KSCN, and NH4Br in N,N-dimethylformamide,” J. Electrochem. Soc.,121, No. 8, 977–982 (1974).
G. A. Krestov and G. I. Kurakina, “Derivatographic investigation of ion coordination in the long-range solvation region,” Zh. Fix. Khim.,40, No. 7, 1910–1913 (1970).
N. I. Gusev, “Investigations of ion hydration by an electrical conductivity method. 10. Changes in the numbers of molecules oriented by cations in the temperature range from 10 to 50°C,” Zh. Fiz. Khim.,47, No. 1, 96–100 (1973).
B. F. J. Vorgin, P. S. Knapp, W. L. Flint, et al., “NMR studies of aqueous electrolyte solutions. 4. Hydration numbers of strong electrolytes determined for temperature effects on proton shifts,” J. Chem. Phys.,54, No. 1, 178–181 (1971).
A. N. Lyashchenko, “Coordination numbers and nature of the structural environment of ions in an aqueous solution,” Zh. Fiz. Khim.,50, No. 11, 2729–2735 (1976).
W. Kemula, J. Malyszko, E. Malyszko, and Z. Kopyra, “Hydration numbers of some cations from viscosity data,” Bull. Acad. Pol.,17, No. 10, 605–608 (1969).
W. C. Cabe and H. F. Fisher, “A near-infrared spectroscopic method for investigating the hydration of a solute in aqueous solution,” J. Phys. Chem.,74, No. 15, 2990–2998 (1970).
E. R. Malinowski, “NMR studies of aqueous electrolyte solutions. 2. Hydration of Al(NO3)3 determined from temperature effects on proton shifts,” J. Chem. Phys.48, No. 11, 4989–4991 (1968).
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya i Experimental'naya Khimiya, Vol. 21, No. 5, pp. 627–631, September–October, 1985.
The author wish to express their gratitude to professors Yu. Ya. Fialkov and M. A. kvadrigin for fruitful discussions of the result of the current work.
Rights and permissions
About this article
Cite this article
Koshkin, V.M., Evtushenko, V.D. & Muraeva, O.A. Relative viscosity and the solvation number in solutions. Theor Exp Chem 21, 600–603 (1985). https://doi.org/10.1007/BF00944103
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00944103