Abstract
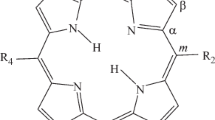
In isotropic media at 4.2°K with selective laser excitation, fine-structure fluorescence spectra have been obtained for NH-tautomers of 2,3,12,13-tetramethylporphine (TMP) and their derivatives in which the central protons have been replaced by deuterons. For each of the phototautomers, polarization measurements have been made for individual background-free lines, and the symmetry of the normal vibrations has been determined. From an analysis of the experimental data and the results obtained in calculating the normal vibrations of the 2,3,12,13-TPM molecule with various possible positions of the imine protons, it has been shown that the phototautomer with fluorescence in the shorter-wave region corresponds to location of the central protons on the pyrrole rings with methyl substituents, and the tautomer with fluorescence in the longer-wave region corresponds to location of the protons on the “porphine“ pyrrole rings. For “porphine“ 2,3,12,13-TMP, and also for 2,3,7,8-TMP and 2,3,7,8-TEP, no phototautomers with adjacent positions of the NH-protons were detected.
Similar content being viewed by others
Literature cited
C. V. Storm and Y. Tekly, “Nitrogen-hydrogen tautomerizm in porphyrins and chlorins,” J. Am. Chem. Soc.,94, No. 5, 1745–1747 (1972).
K. N. Solov'ev, I. E. Zalesskii, V. N. Kotlo, and S. F. Shkirman, “Photoinduced interconversions of centers responsible for ‘multiplicity’ in Shpolsky effect,” Pis'ma Zh. Éksp. Teor. Fiz.,17, No. 9, 463–466 (1973).
E. I. Zenkevich, A. M. Shulga, A. V. Chernook, and G. P. Gurinovich, “Spectral peculiarities of NH-tautomerism in isocycle-containing porphyrins and their covalently linked dimers,” Chem. Phys. Lett.,109, No. 3, 306–311 (1984).
A. I. M. Dicker, M. Noort, H. P. H. Thijssen, et al., “Zeeman effect of the S1 ← So transition of the two tautomeric forms of chlorin: a study by photochemical hole burning in an n-hexane host at 4.2°K,” Chem. Phys. Lett.,78., No. 2, 212–218 (1981).
L. L. Gladkov and K. N. Solov'ev, “Force field for in-plane vibrations of porphine molecule,” Zh. Prikl. Spektrosk.,40, No. 2, 275–284 (1984).
A. S. Starukhin, “Investigation of vibrational and fine-structure vibronic spectra of porphyrin macrocycles,” Author's Abstract of Candidate's Dissertation, Minsk (1910).
A. N. Shul'ga, L. L. Gladkov, I. V. Stanishevskii, and A. S. Starukhin, “Fine-structure fluorescence spectra of isomers of Zn-tetramethylporphyrin with selective laser excitation, at 4.2°K,” Teor. Éksp. Khim.,21, No. 4, 431–439 (1985).
A. S. Starukhin, A. N. Shul'ga, and I. V. Stanishevskii, “Method for obtaining fine-structure fluorescence spectra of NH-tautomers of substituted porphyrins in solid solutions,” Opt. Spektrosk.,58, No. 4, 936–939 (1985).
C. La Lau and R. G. Snyder, “A valence force field for alkylbenzenes. Toluene, pxylene, m-xylene, mesitylene, and some of their deuterated analogs,” Spectrochim. Acta A,27, No. 10, 2073–2088 (1971).
B. M. L. Chen and A. Tulinsky, “Redetermination of the structure of porphine,” J. Am. Chem. Soc.,94, No. 12, 4144–4151 (1972).
J. W. Lauher and J. A. Ibers, “Structure of octaethylporphyrins. A comparison with other free base porphyrins,” J. Am. Chem. Soc.,95, No. 16, 5148–5152 (1973).
G. D. Egorova, K. N. Solov'ev, and A. M. Shul'ga, “PMR spectra of symmetric meso-substituted porphyrins and chlorins,” Teor. Éksp. Khim.,11, No. 1, 77–86 (1975).
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya i Éksperimental'naya Khimiya, Vol. 21, No. 5, pp. 554–560, September–October, 1985.
Rights and permissions
About this article
Cite this article
Shul'ga, A.M., Gladkov, L.L., Stanishevskii, I.V. et al. Investigation of NH-tautomerism of alkyl derivatives of porphine by means of low-temperature selective excitation spectroscopy. Theor Exp Chem 21, 529–535 (1985). https://doi.org/10.1007/BF00944086
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00944086