Abstract
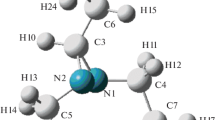
A plant for the theoretical analysis of the structure and intramolecular dynamics of ion pairs of organic salts based on the combined use of the results of quantum-chemical calculations and molecular mechanics has been proposed. The potential-energy surfaces of the interionic interactions in imidazolium halide and N-methylimidazolium halide ion pairs have been investigated, and the equilibrium geometric parameters and frequencies of the interionic vibrations have been determined. It has been established that these ion pairs should be classified as stereochemically nonrigid molecules.
Similar content being viewed by others
Literature cited
M. Szwarc (editor), Ions and Ion Pairs in Organic reactions, Vol. 1, Wiley-Interscience, New York (1972).
J. E. Gordon, The Organic Chemistry of Electrolyte Solutions, Wiley-Interscience, New-York (1975).
A. A. Solov'yanov and I. P. Beletskaya, “Effects of ionic association in organic chemistry,” in: Physical Chemistry. Contemporary Problems [in Russian], Khimiya, Moscow (1980), pp. 247–283.
J. Kuthan, S, Böhm, and V. Skála, “On the applicability of EHT method for study of interactions of pyridinium ions with anions. Calculations on pyridine hydrochloride,” Collect. Dzech. Chem. Commun.,44, No. 1, 99–109 (1979).
P. A. Kollman and D. M. Hayes, “Theoretical calculations on proton-transfer energetics: studies of methanol, imidazole, formic acid, and methanethiol as models for the serine and cysteine proteases,” J. Am. Chem. Soc.,103, No. 11, 2955–2961 (1981).
F. Zuccarello, A. Raudino, and G. Buemi, “A theoretical study of ion pairs in aqueous solution. Binding of cholinergic neuro transmitters to the anionic sites of synaptic receptors,” J. Mol. Struct.,87, No. 2, 197–204 (1982).
V. G. Dashevskii, Conformational Analysis of Organic Molecules [in Russian], Khimiya, Moscow (1982).
L. M. Litvinenko, V. A. Dadali, S. A. Lapshin, et al., “Mechanism for the benzoylation of imidazole derivatives,” Zh. Org. Khim.,11, No. 2, 249–256 (1975).
S. A. Lapshin, V. A. Dadali, Yu. S. Simanenko, and L. M. Litvinenko, “Investigation of the kinetics of the interaction of acetyl halides with aromatic amines in the presence of N-methylimidazole,” Zh. Org. Khim.,13, No. 3, 586–594 (1977).
S. A. Lapshin, V. A. Dadali, L. M. Litvinenko, and Yu. S. Simanenko, “Investigation of the mechanism of the interaction of acetyl halides with aromatic amines in the presence of N-substituted imidazoles,” Zh. Org. Khim.,17, No. 9, 1938–1944 (1981).
V. A. Dadali, E. V. Titov, Yu. S. Simanenko, et al., “IR spectra and structure of some N-acylimidazolium salts,” Ukr. Khim. Zh.,42, No. 6, 598–603 (1976).
E. V. Titov and V. I. Rybachenko, “Molecular spectroscopy of interionic interaction and the structure of ion pairs (ammonium salts),” J. Mol. Struct.,60, No. 1, 67–72 (1980).
V. I. Rybachenko, V. N. Marchenko, A. I. Pletnev, et al., “Dielectric losses of the N-methylimidazole-acetyl chloride system in dichloromethane,” Ukr. Khim. Zh.,44, No. 12, 1307–1310 (1978).
K. Kuchitsu, “Geometrical parameters of free molecules: their definitions and determination by gas electron diffraction,” in: Diffraction Studies on Non-crystalline Substances, Akad. Kiado, Budapest (1981), pp. 63–116.
R. J. Abraham and K. Parry, “Rotational isomerism. 8. A calculation of the rotational barriers and rotamer energies of some halogenated compounds,” J. Chem. Soc., B, No. 3, 539–545 (1970).
C. R. A. Catlow and W. C. Wackrodt, Computer Simulation of Solids. Lecture Notes in Physics Springer, New York (1982).
T. L. Hill, “Steric effects. 1. van der Waals potential energy curves,” J. Chem. Phys.,16, No. 4, 399–404 (1948).
F. A. Momany, R. F. McGuire, A. W. Burgess, and H. A. Scheraga, “Energy parameters in polypeptides. 7. Geometric parameters, partial atomic charges, nonbonded interactions, hydrogen bond interactions, and intrinsic torsional potential for the naturally occurring amino acids,” J. Phys. Chem.,79, No. 22, 2361–2381 (1975).
A. T. Hagler, E. Huler, and S. Lifson, “Energy functions for peptides and proteins. 1. Derivation of a consistent force field including the hydrogen bond from amide crystals,” J. Am. Chem. Soc.,96, No. 17, 5319–5327 (1974).
R. C. Bingham, M. J. S. Dewar, and D. H. Lo, “Ground states of molecules. 25. MINDO/3. An improved version of the MINDO semiempirical SCF-MO method,” J. Am. Chem. Soc.,97, No. 6, 1285–1293 (1975).
J. A. Pople and M. Gordon, “Molecular orbital theory of the electronic structure of organic compounds. 1. Substituent effects and dipole moments,” J. Am. Chem. Soc.,89, No. 17, 4253–4261 (1967).
R. H. Stokes, “The van der Waals radii of gaseous ions of the noble gas structure in relation to hydration energies,” J. Am. Chem. Soc.,86, No. 6, 979–982 (1964).
N. G. Rambidi, S. M. Tolmachev, G. I. Gurova, et al., Tables of Standard Reference Data. Geometric Configuration of Nuclei and Internuclear Distances of Molecules and Ions in the Gaseous Phase [in Russian], Izd. Standartov, Moscow (1978).
H. M. Seip, “Studies of molecules with five-membered rings. 1. Calculation of conformational energies for tetrahydrofuran and 1, 2, 4-trioxacyclopentane,” Acta Chem. Scand.,23, No. 8, 2741–2747 (1969).
D. M. Himmelblau, Applied Nonlinear Programming, McGraw-Hill, New York (1972).
V. P. Spiridonov, A. A. Ishchenio, and E. Z. Zasorin, “Investigation of stereochemically nonrigid molecules by electron diffraction analysis,” Usp. Khim.,47, No. 1, 101–126 (1978).
L. M. Litvinenko, S. A. Lapshin, A. Yu. Chervinskii, et al., “Investigation of intermediate products of nucleophilic catalysis in media of low polarity by PMR,” Dokl. Akad. Nauk SSSR,270, No. 6, 1401–1403 (1983).
V. I. Rybatchenko and E. V. Titov, “Spectroscopy of molecular interactions with acyl transport,” J. Mol. Struct.,47, No. 1, 65–82 (1978).
E. V. Titov, V. I. Rybachenko, L. D. Goncharova, and R. G. Semenova “Study of the ionic association of quaternary-nitrogen salts. N-Acetylammonium salts in acetonitrile,” Zh. Fiz. Khim.,54, No. 12, 3099–3103 (1980).
R. G., Semenova, V. I. Rybachenko, and E. V. Titov, “Study of the exchange reaction between 4-dimethylaminopyridine, N-methylimidazole, and their N-acyl salts in CH3CN,” Teor. Éksp. Khim.,20, No. 2, 250–254 (1984).
B. I. Zhilinskii, V. A. Istomin, and N. F. Stepanov, “Vibration-rotation states of nonrigid molecules,” Sovrem. Probl. Fiz. Khim.,11, 259–304 (1979).
E. V. Titov, K. Yu. Chotii, and V. I. Rybachenko, “Far-infrared spectra and characteristics of the ionic bonding in N-hydroxypyridinium salts,” Teor. Éksp. Khim.,19, No. 6, 761–764 (1983).
Author information
Authors and Affiliations
Additional information
Deceased.
Translated from Teoreticheskaya i Éksperimental'naya Khimiya, Vol. 21, No. 5, pp. 522–529, September–October, 1985.
Rights and permissions
About this article
Cite this article
Litvinenko, L.M., Fershikov, A.G., Panchenko, B.V. et al. Theoretical analysis of the structure and intramolecular dynamics of imidazolium halide and N-methylimidazolium halide ion pairs. Theor Exp Chem 21, 497–504 (1985). https://doi.org/10.1007/BF00944081
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00944081