Abstract
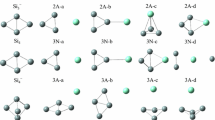
The electronic configuration of the HOSiO3 3− cluster, which simulates an isolated OH group on an SiO2 surface, has been calculated by the SCF-Xα-scattered-wave method with overlapping spheres. The calculation of the binding energies of the Si core electrons confirmed the previously drawn conclusion [see Teor. Éksp. Khim.,16, No. 4, 458 (1980)] that the formation of pairs of ions (≡Si+, ≡SiO−) and radicals (≡Si·, ≡SiO·) upon the dehydroxylation of SiO2 should be accompanied by negative chemical shifts of E2s and E2p of Si, while the formation of di- and trisiloxane bridges should be accompanied by positive chemical shifts. A comparison of the values of E2s and E2p of Si for various clusters was conducted with the use of corrections which take into account the differences in the radii of the Watson spheres. Extrapolation of the dependence of qSi on E2s and E2p of Si revealed that the charge of Si in crystalline silicon is equal to 0.6–0.8. The height of the barrier to the hindered rotation of a surface OH group on SiO2 calculated in the framework of the model adopted is equal to 0.052 eV, which is in good agreement with the experimental value (0.038 eV).
Similar content being viewed by others
Literature cited
A. V. Kiselev and V. I. Lygin, Infrared Spectra of Surface Compounds [in Russian], Nauka, Moscow (1972).
B. N. Laskorin, V. V. Strelko, D. N. Strazhesko. and V. I. Denisov. Sorbents Based on Silica Gel in Radiochemistry [in Russian], Atomizdat, Moscow (1977).
K. B. Yatsimirskii, V. V. Nemoshkalenko, A. A. Chuiko, et al., “Investigation of adsorption complexes on silica surfaces by x-ray photoelectron spectroscopy,” Metallofizika, No. 60, 60–67 (1975).
V. M. Ogenko, “Investigation of the nature of active sites on the surfaces of dispersed silicas,” Author's abstract of dissertation for the degree of Candidate of Chemical Sciences, Kiev (1974).
K. Siegbahn, C. Nordling, A. Fahlman, R. Nordberg, K. Hamrin, J. Hedman, G. Johansson, T. Bergmark, S. Karlsson, I. Lindgren, and B. Lindberg, ESCA — Electron Spectroscopy for Chemical Analysis, McPherson Instrument Corp., Acton, Mass. (1967).
V. I. Nefedov, Application of X-Ray Photoelectron Spectroscopy in Chemistry (Results of Science and Technology, Molecular Structure and Chemical Bonding Series, Vol. 1) [in Russian], VINITI, Moscow (1973).
A. G. Guzikevich, Yu. I. Gorlov, and A. A. Chuiko, “Application of the Xα-SW method to the study of the structure of a dehydroxylated silica surface,” Teor. Éksp. Khim.,16, No. 4, 458–464 (1980).
K. H. Johnson, “‘Multiple scattering’ model for polyatomic molecules,” J. Chem. Phys.,45, No. 8, 3085–3095 (1966).
J. C. Slater and K. H. Johnson, “Self-consistent-field Xα cluster method for polyatomic molecules and solids,” Phys. Rev., B,5, No. 3, 844–853 (1972).
J. B. Perry, “Infrared study of OH and NH2 groups on the surface of a dry silica aerogel,” J. Phys. Chem.,70, No. 9, 2937–2945 (1966).
K. Schwartz, “Optimization of the statistical exchange parameter α for the free atoms H through Nb,” Phys. Rev., B,5, No. 7, 2466–2468 (1975).
J. G. Norman, “Nonempirical versus empirical choices for overlapping sphere radii ratios in SCF-X-SW calculations on ClO4 − and SO2,” Mol. Phys.,31, No. 4, 1091–1098 (1976).
K. E. Watson, “Analytical Hartree-Fock solutions for O−,” Phys. Rev.,111, No. 4, 1108–1110 (1958).
T. Bernstein, H. Ernst, D. Freude, and I. Jünger, “NMR-Untersuchungen an Kieselgelhydroxylgruppen,” Z. Phys. Chem.,262, No. 6, 1123–1134 (1981).
P. R. Ryason and B. G. Russel, “An infrared study of isolated hydroxyl groups on silica surfaces,” J. Phys. Chem.,79, No. 3, 1276–1279 (1975).
G. V. Gadiyak, V. G. Malkin, Yu. N. Morokov, and S. F. Ruzankin, “Calculation of the Ge2 molecule by the Xα-SW method,” Zh. Strukt. Khim.,22, No. 2, 38–42 (1981).
K. H. Johnson, “Quantum chemistry,” Ann. Rev. Phys. Chem.,26, 39–57 (1975).
U. Wahlgren, “Calculation of potential barriers using the SCF-Xα-SW method. NH3 and H2O2,” Chem. Phys. Lett.,79, No. 13, 246–250 (1975).
S. G. Gagarin, G. M. Zhidomirov, Yu. V. Plekhanov, and I. N. Senchenya, “Quantum-chemical study of the energy spectrum of a catalyst surface. 3. Electronic structure of a model electron-acceptor site on an SiO2 surface,” Zh. Strukt. Khim.,22, No. 2, 15–21 (1981).
L. A. Mai, “Atomic charge of silicon in a crystal lattice,” Izv. Akad. Nauk Latv. SSR, Ser. Khim., No. 5, 629 (1983).
G. M. Zhidomirov and I. D. Mikheikin, Cluster Approximation in Quantum-Chemical Investigations of Chemisorption and Surface Structures (Results of Science and Technology, Molecular Structure and Chemical Bonding Series, Vol. 9) [in Russian], VINITI (1984).
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya i Éksperimental'naya Khimiya, Vol. 21, No. 5, pp. 513–522, September–October, 1985.
Rights and permissions
About this article
Cite this article
Guzikevich, A.G. Calculations of the chemical shifts of E2s and E2p of Si in model clusters of an SiO2 surface. Theor Exp Chem 21, 489–497 (1985). https://doi.org/10.1007/BF00944080
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00944080