Abstract
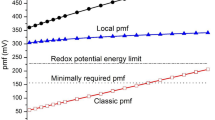
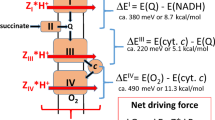
The\(\Delta \bar \mu H^ + \) and the ΔGp have been measured in whole cells ofMethylophilus methylotrophus during the oxidation of various respiratory chain substrates. The magnitude of the\(\Delta \bar \mu H^ + \) depended on the external pH and the composition of the assay medium, and varied from-109 to-165 mV. The relative contributions of the ΔΨ and the ΔpH to the\(\Delta \bar \mu H^ + \) were found to vary with the external pH such that the internal pH remained constant; depending on the composition of the assay medium, this value was between 6.6 and 7.0. A ΔGp of approximately-46 kJ/mol was generated during the oxidation of methanol, and either the ΔΨ or ΔpH alone was fully competent to drive ATP synthesis. Respiration and ATP synthesis were found to be poised far from equilibrium under the conditions of these experiments, and the value of the ΔGp was thus controlled kinetically. Comparison of the\(\Delta \bar \mu H^ + \) with the ΔGp yielded an →H+/ATP quotient >2.6 g-ion H+/mol ATP.
Similar content being viewed by others
Abbreviations
- TMPD:
-
N,N,N′,N′-tetramethyl-p-phenylenediamine
- FCCP:
-
carbonylcyanidep-trifluoromethoxyphenylhydrazone
- DMO:
-
5,5′-dimethyloxazolidine-2,4-dione
- TPMP+ :
-
triphenylmethylphosphonium (iodide salt); Tween 20, polyoxyethylenesorbitan monolaurate
- TPP+ :
-
tetraphenylphosphonium (bromide salt)
- \(\Delta \bar \mu H^ + \) :
-
bulk phase transmembrane electrochemical potential difference of protons (\(\bar \mu H_{^{in} }^ + - \bar \mu H_{^{out} }^ + \))
- ΔpH:
-
bulk phase transmembrane pH difference (pHin-pHout)
- ΔΨ:
-
bulk phase transmembrane electrical potential difference (Ψin-Ψout)
- Δp:
-
true protonmotive force (incorporating both bulk phase and localised protons;\(\Delta \bar \mu H^ + \))
References
Anthony C (1981) Electron transport in methylotrophic bacteria. In: Dalton H (ed) Microbial growth on C1 compounds. Heyden, London, pp 220–230
Atkinson DE (1968) The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 7:4030–4034
Beardsmore AJ, Quayle JR (1978) Carbon assimilation by methanolgrownMethylophilus methylotrophus. Proc Soc Gen Microbiol 5: 41–42
Bernt E, Bergmeyer HU (1974) Acetaldehyde determination with alcohol dehydrogenase from yeast. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic, London, pp 1506–1507
Branca D, Ferguson SJ, Sorgato MC (1981) Clarification of factors influencing the nature and magnitude of the protonmotive force in bovine heart submitochondrial particles. Eur J Biochem 116:341–346
Brooks JD, Meers JL (1973) The effect of discontinuous methanol addition on the growth of a carbon-limited culture of Pseudomonas. J Gen Microbiol 77:513–519
Collins SH, Hamilton WA (1976) Magnitude of the protonmotive force in respiringStaphylococcus aureus andEscherichia coli. J Bacteriol 126:1224–1231
Cross AR, Anthony C (1980) The electron transport chains of the obligate methylotrophMethylophilus methylotrophus. Biochem J 192:429–439
Dawson MJ, Jones CW (1981a) Respiration-linked proton translocation in the obligate methylotrophMethylophilus methylotrophus. Biochem J 194:915–924
Dawson MJ, Jones CW (1981b) Chemiosmotic aspects of respiratory chain energy conservation inMethylophilus methylotrophus. In: Dalton H (ed) Microbial growth on C1 compounds. Heyden, London, pp 251–257
Dawson MJ, Jones CW (1981c) Energy conservation in the terminal region of the respiratory chain of the methylotrophic bacteriumMethylophilus methylotrophus. Eur J Biochem 118:113–118
Duine JA, Frank J, Verwiel PEJ (1980) Structure and activity of the prosthetic group of methanol dehydrogenase. Eur J Biochem 108: 187–192
Erecinska M, Davis JS, Wilson DF (1979) Regulation of respiration inParacoccus denitrificans; the dependence of redox state of cytochromec and [ATP]/[ADP][Pi]. Arch Biochem Biophys 197:463–469
Friedberg I, Kaback HR (1980) Electrochemical proton gradient inMicrococcus lysodeikticus cells and membrane vesicles. J Bacteriol 142:651–658
Häggström MH, Dostalek M (1981) Growth ofMethylomonas methanolica: factors influencing growth yield. Europ J Appl Microbiol Biotechnol 12:107–112
Hopner T, Knappe J (1974) Formate determination with formate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic, London, pp 1551–1555
Kell DB (1979) On the functional proton current pathway of electron transport phosphorylation. Biochim Biophys Acta 549:55–99
Kell DB, Ferguson SJ, John P (1978a) Measurement by a flow dialysis technique of the steady state protonmotive force in chromatophores fromRhodospirillum rubrum. Comparison with phosphorylation potential. Biochim Biophys Acta 502:111–126
Kell DB, John P, Ferguson SJ (1978b) The protonmotive force in phosphorylating membrane vesicles fromParacoccus denitrificans. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J 174:257–266
Kell DB, Peck MW, Rodger G, Morris JG (1981) On the permeability to weak acids and bases of the cytoplasmic membrane ofClostridium pasteurianum. Biochem Biophys Res Commun 99:81–88
Linton JD, Griffiths K, Gregory M (1981) The effect of mixtures of glucose and formate on the yield and respiration of a chemostat culture ofBeneckea natriegens. Arch Microbiol 129:119–122
McCarthy JEG, Alefounder PR, Ferguson SJ (1980) Mechanistic aspects of aerobic and anaerobic oxidative phosphorylation inParacoccus denitrificans. 1st European Bioenergetics Conference, Short Reports, pp 161–162. Patron Editore, Bologna
McCarthy JEG, Ferguson SJ, Kell DB (1981) Estimation with an ionselective electrode of the membrane potential in cells ofParacoccus denitrificans from the uptake of butyltriphenylphosphonium cation during aerobic and anaerobic respiration. Biochem J 196:311–321
McKay AM, Quilter JA, Jones CW (1982) Energy conservation in the extreme thermophileThermus thermophilus HB 8. Arch Microbiol 131:43–50
Michel H, Oesterhelt D (1980) Electrochemical proton gradient across the cell membrane ofHalobacterium halobium. Biochemistry 19: 4607–4614
Murastugu M, Kamo N, Kobatake Y, Kimura K (1979) Determination of membrane potential forEscherichia coli with use of an electrode sensitive to tetraphenylphosphonium. J Electroanalyt Chem 104: 477–491
Nicholls DG (1974) The influence of respiration and ATP hydrolysis on the proton electrochemical gradient across the inner membrane of rat liver mitochondria as determined by ion distribution. Eur J Biochem 50:305–315
Ramos S, Schuldinger S, Kaback HR (1979) The use of flow dialysis for determinations of ΔpH and active transport. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 55. Academic, London, pp 680–688
Rosing J, Slater EC (1972) The value of ΔG° for the hydrolysis of ATP. Biochim Biophys Acta 267:275–290
Rottenberg H (1979) The measurement of membrane potential and ΔpH in cells, organelles and vesicles. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. vol 55. Academic, London. pp 547–569
Salisbury SA, Forrest HS, Cruse WBT, Kennard O (1979) A novel coenzyme from bacterial primary alcohol dehydrogenases. Nature (London) 280:843–844
Sorgato MC, Ferguson SJ, Kell DB, John P (1978) The protonmotive force in bovine heart submitochondrial particles. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J 174:237–256
Strom T, Ferenci T, Quayle JR (1974) The carbon assimilation pathways ofMethylococcus capsulatus, Pseudomonas methanica andMethylosinus trichosporium (OB3B) during growth on methane. Biochem J 144:465–476
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dawson, M.J., Jones, C.W. The protonmotive force and phosphorylation potential developed by whole cells of the methylotrophic bacteriumMethylophilus methylotrophus . Arch. Microbiol. 133, 55–61 (1982). https://doi.org/10.1007/BF00943770
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00943770