Conclusions
-
1.
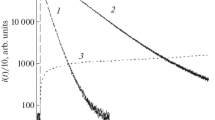
A mechanism describing the quantitative aspects of the chemiluminescence in the interaction of luminol with tetranitromethane in the presence of H2O2 has been proposed. The particles responsible for the chemiluminescence are active intermediates resulting from reaction of H2O2 with the tetranitromethane.
-
2.
This mechanism has been used to calculate rate constant ratios for reaction of the intermediate with luminol and hydrogen peroxide, and for reaction of the intermediate with luminol and tetranitromethane.
-
3.
The yield of the intermediate which oxidizes the LH2 is 0.5. Yields have been calculated for the excited particles produced in the formation of the emitter.
Similar content being viewed by others
Literature cited
T. P. Vorob'eva, Yu. N. Kozlov, Yu. V. Koltypin, A. P. Purmal', and B. A. Rusin, Izv. Akad. Nauk SSSR, Ser. Khim., 1996 (1978).
T. P. Vorob'eva, Yu. N. Kozlov, Yu. V. Koltypin, A. P. Purmal', and B. A. Rusin, Zh. Fiz. Khim.,52, 1047 (1978).
B. A. Rusin, V. N. Emokhonov, E. L. Frankevich, and V. L. Tal'roze, Khim. Vys. Énerg.,10, 95 (1976).
T. Majiwara and D. R. Kearns, J. Am. Chem. Soc.,95, 5886 (1973).
A. D. Nadezhdin, Yu. N. Kozlov, and A. P. Purmal', Zh. Fiz. Khim.,49, 2263 (1975).
J. B. C. Matheson and J. Lee, Photochem. Photobiol.,24, 605 (1976).
F. H. Stross and G. E. K. Branch, J. Org. Chem.,3, 385 (1938).
E. H. White and M. M. Burseu, J. Am. Chem. Soc.,86, 941 (1964).
J. Lee and H. H. Seliger, Photochem. Photobiol.,11, 247 (1970).
B. A. Rusin, V. N. Emokhonov, E. L. Frankevich, and V. L. Tal'roze, Dokl. Akad. Nauk SSSR,221, 1371 (1973).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 12, pp. 2715–2718, December, 1978.
Rights and permissions
About this article
Cite this article
Vorob'eva, T.P., Koztov, Y.N., Koltypin, Y.V. et al. Luminol oxidation with chemiluminescence 6. The mechanism of chemiluminescence of the luminol-CNO2)4-H2O2 system. Russ Chem Bull 27, 2423–2426 (1978). https://doi.org/10.1007/BF00941089
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00941089