Abstract
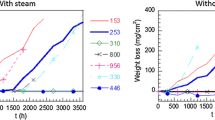
Fe-20Cr-5Al alloy foils are used in automotive catalytic converters. This work examines oxidation behavior of four production-processed alloy foils in both air and synthetic exhaust gas environments. Oxidation tests were performed between 750° C and 1150° C for times to 96 hrs. Weight gain results in both atmospheres were similar, an indication that the same mechanism controls oxidation in both environments. At high temperatures (>-950° C) both atmospheres produce weight gains consistent with α-alumina growth. Activation energies of 323 kJ/gmole and 271 kJ/gmole were calculated for oxidation in air and synthetic exhaust gas, respectively. At lower temperatures (<-850° C), accelerated weight gains can occur from growth of transition alumina. Despite similar weight gain results, the two atmospheres produce different oxide morphologies: at 950° C and above, air produces a rounded, porous oxide while synthetic exhaust produces a more compact, angular oxide. Unexpectedly, oxide spalling occurred on foils oxidized in synthetic exhaust at 1050° C and above.
Similar content being viewed by others
References
E. A. Gulbransen and K. F. Andrew,J. Electrochem. Soc. 106, 294 (1959).
C. S. Wukusick and J. F. Collins,Mat. Res. Standards 4, 637 (1964).
W. C. Hagel,Corrosion 21, 316 (1965).
T. Amano, S. Yajima, and Y. Saito,Trans. Japan Inst. Metals 20, 431(1979).
B. Pieraggi,Oxid. Met. 27, 177 (1987).
Joint Committee for Powder Diffraction Standards Entry 35-121.
K. Wefers and G. M. Bell, Alcoa Research Laboratories Technical Paper No. 19 (1972).
G. C. Rybicki and J. L. Smialek,Oxid. Met. 31, 275 (1989).
P. T. Moseley, K. R. Hyde, B. A. Bellamy, and G. Tappin,Corros. Sci. 24, 547 (1984).
Y. Oishi and W. D. Kingery,J. Chem. Phys. 33, 480 (1960).
A. E. Paladino and W. D. Kingery,J. Chem. Phys. 37, 957 (1962).
H. A. Wang and F. A. Kroger,J. Am. Ceram. Soc. 63, 613 (1980).
J. K. Tien and F. S. Pettit,Metallurg. Trans. 3, 1587 (1972).
T. A. Ramanarayanan, M. Raghavan, and R. Petkovic-Luton,J. Electrochem. Soc. 131, 923 (1984).
F. A. Golightly, F. H. Stott, and G. C. Wood,Oxid. Met. 10, 163 (1976).
W. J. Quadakkers, H. Holzbrecher, K. G. Briefs, and H. Beske,Oxid. Met. 32, 67 (1989).
G. Ben Abderrazik, G. Moulin, and A. M. Huntz,Solid State Ionics 22, 285 (1987).
K. P. R. Reddy, J. L. Smialek, and A. R. Cooper,Oxid. Met. 17, 429 (1982).
E. W. A. Young and J. H. W. De Wit,Solid State Ionics 16, 39 (1985).
J. Jedlinski and S. Mrowec,Mat. Sci. Engin. 87, 281 (1987).
A. W. Funkenbusch, J. G. Smeggil, and N. S. Bornstein,Metallurg. Trans. 16A, 1164 (1985).
J. G. Smeggil, A. W. Funkenbusch, and N. S. Bornstein,Metallurg. Trans. 17A, 923 (1986).
J. L. Smialek,Metallurg. Trans. 18A, 164 (1987).
D. R. Sigler,Oxid. Met. 29, 23 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sigler, D.R. Oxidation behavior of Fe-20Cr-5Al rare earth alloys in air and synthetic exhaust gas. Oxid Met 36, 57–80 (1991). https://doi.org/10.1007/BF00938456
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00938456