Abstract
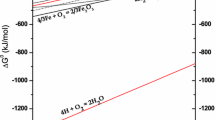
High-purity iron has been oxidized at 1000–1200° C in CO2 and in CO2 + CO with different compositions and at different total gas pressures (0.1–1 atm.). The experimental work has comprised thermogravimetric reaction rate measurements and characterization of the wüstite scales by metallography and x-ray diffraction. The overall results have been analyzed in terms of a classical model for coupled linear/parabolic kinetics, where it is assumed that the surface of growing wüstite scales has exactly the same defect structure and defect concentrations as that of bulk wüstite equilibrated in the same gaseous atmospheres. Important discrepancies are found between the predicted and the experimentally observed reaction behavior. Thus, both the linear and parabolic rate constants are found to be dependent on the partial pressure of CO2 and the total gas pressure of the CO2 + CO gas mixtures, and furthermore, the reaction in CO2 + CO is slower than in O2 and in H2O + H2 with the same oxygen activity. In order to explain the experimental results, it is suggested that CO and CO2 molecules interact with the wüstite surface and thereby affect the defect structure and defect concentrations in a thin surface layer, and that this, in turn, affects both the linear and parabolic reaction rates.
Similar content being viewed by others
References
K. Hauffe and H. Pfeiffer,Z. Metallk. 44, 27 (1953).
W. W. Smeltzer,Acta Met. 8, 377 (1960).
F. S. Pettit, R. Yinger, and J. B. Wagner, Jr.,Acta Met. 8, 617 (1960).
F. S. Pettit and J. B. Wagner, Jr.,Acta Met. 12, 35 (1964).
K. Hedden and G. Lehmann,Arch. Eisenhüttenwesen 35, 839 (1964).
L. A. Morris and W. W. Smeltzer,Acta Met. 15, 1591 (1967).
E. T. Turkdogan and J. V. Vinters,Met. Trans. 3, 1561 (1972).
S. M. El Rahgy, F. Jeannot, and C. Gleitzer,J. Mater. Sci. Lett. 13, 2510 (1978).
R. Bredesen and Per Kofstad,Oxid. Met. 34, 361 (1990).
R. Bredesen and Per Kofstad,Oxid. Met. 35, 107 (1991).
W. W. Smeltzer,Trans. Met. Soc. AIME 218, 674 (1960).
C. Wagner,Ber. Bunsenges. 70, 775 (1966).
L. S. Darken and R. W. Gurry,J. Am. Ceram. Soc. 67, 1398 (1945).
P. Vallet and P. Raccah,Mem. Sci. Rev. Met. 62, 1 (1965).
R. J. Ackermann and R. W. Sandford, Tech. Rept. ANL-7250 (September 1966), p. 46.
B. Swaroop and J. B. Wagner, Jr.,Trans. AIME 239, 1215 (1967).
H. G. Sockel and H. Schmalzried,Ber. Bunsenges. Phys. Chem. 72, 745 (1968).
R. A. Giddings and R. S. Gordon,J. Am. Ceram. Soc. 56, 111 (1973).
H.-J. Grabke,Ber. Bunsenges. Phys. Chem. 69, 48 (1965).
B. Pieraggi,Oxid. Met. 27, 177 (1987).
P. Kofstad, inHigh Temperature Corrosion (Elsevier, New York, 1988).
E. T. Turkdogan, W. M. McKewan, and L. Zwell,J. Phys. Chem. 69, 327 (1965).
F. Nardou, P. Raynaud, and M. Billy,J. Chim. Phys. 76, 595 (1979).
R. L. Levin and J. B. Wagner, Jr.,Trans. Met. Soc. AIME 233, 159 (1965).
L. W. Laub and J. B. Wagner, Jr.,Oxid. Met. 7, 1 (1973).
F. Millot and J. Berthon,J. Phys. Chem. Solids 47, 1 (1986).
A. Sadowski, G. Petot-Ervas, C. Petot, and J. Janowski,Proc. of the Third Round Table Meeting on Physico-Chemical and Structural Properties and Kinetics of Reduction of Wüstite and Magnetite (Sept. 28–Oct. 3 1986, Jadwisin, Poland), inMetalurgia I Odlewnictwo, p. 259.
E. Riecke and K. Bohnenkamp,Arch. Eisenhüttenw. 40, 717 (1969).
C. Carel and J. R. Gavarri,Mater. Res. Bull. 11, 745 (1976).
R. L. Levin and J. B. Wagner, Jr.,Trans. Met. Soc. AIME 236, 516 (1966).
E. R. Jette and F. Foote,J. Chem. Phys. 1, 29 (1933);AIME Trans. 105, 276 (1933).
B. Touzelin,Proc. of the Third Round Table Meeting on Physico-Chemical and Structural Properties and Kinetics of Reduction of Wüstite and Magnetite (Sept. 28–Oct. 3 1986), Jadwisin, Poland, inMetalurgia I Odlewnictwo, p. 107.
F. Freund, G. Debras, and G. Demortier,J. Am. Ceram. Soc. 61, 429 (1978).
H. Wengler, R. Knobel, H. Katherin, G. Demortier, G. Wolff, and F. Freund,J. Phys. Chem. Solids 43, 59 (1982).
H. Katherin and F. Freund,J. Phys. Chem. Solids 44, 177 (1983).
H. Katherin, H. Gonska, and F. Freund,J. Appl. Phys. A30, 33 (1983).
F. Freund,Proc. of “Science of Ceramics 13” (Orléans, France, 1985), publ. inThe Journal de Physique 1986, P. Odier, F. Cabannes, and B. Cales, eds., p. 499.
J. Nowotny,Mater. Sci. Forum 29, 99 (1988).
J. Nowotny, inSurfaces and Interfaces of Ceramic Materials (1989), p. 205.
R. G. Egdell and W. C. Mackrodt, inSurfaces and Interfaces of Ceramic Materials (1989), p. 185.
J. M. Blakely and S. M. Mukhopadhyay, inSurfaces and Interfaces of Ceramic Materials (1989), p. 285.
R. G. Egdell and W. C. Mackrodt,J. Am. Ceram. Soc. 72, 1576 (1989).
I. Wolf and H.-J. Grabke,Solid State Commun. 54, 5 (1985).
R. S. Roth,Solid State Chem. 13, 159 (1980).
L. Himmel, R. F. Mehl, and C. E. Birchenall,Trans. AIME 197, 827 (1953).
P. Desmaescaux, J. P. Boquet, and P. Lacombe,Bull. Soc. Chim. Fr. 15, 1106 (1965).
P. Hembree and J. B. Wagner, Jr.,Trans. Met. Soc. AIME 245, 1547 (1969).
W. K. Chen and N. L. Peterson,J. Phys. Chem. Solids 36, 1097 (1975).
R. Bredesen and P. Kofstad,Proc. of The Third Round Table Meeting on Physico-Chemical and Structural Properties and Kinetics of Reduction of Wüstite and Magnetite (Sept. 28–Oct. 3 1986), Jadwisin, Poland, inMetalurgia I Odlewnictwo, p. 225.
T. Norby, inSelected Topics in High Temperature Chemistry, (Ø. Johannesen and A. Andersen, eds. (Elsevier, Amsterdam, 1989), p. 101.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bredesen, R., Kofstad, P. On the oxidation of iron in CO2 + CO mixtures. III: Coupled linear parabolic kinetics. Oxid Met 36, 25–56 (1991). https://doi.org/10.1007/BF00938455
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00938455