Abstract
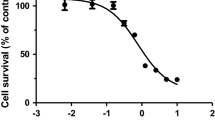
The aim of the research was to study the role played by extracellular O .-2 radicals, which are implicated in cardiac cell damage and the protective effect by cell-permeable, nitroxide, superoxide dismutase-mimics. Cardiomyocytes cultures from 1-day-old rats served as the test-system. Experiments were performed since 5th day in culture when >80% of the cells were beating myocardial cells. Oxidative damage was induced by 0.5 mM hypoxanthine and 0.06 U/ml xanthine oxidase or by 10 mM glucose and 0.15 U/ml glucose oxidase. The parameters used to evaluate damages were spontaneous beating, lactate dehydrogenase release and ATP level. The rhythmic pulsation was followed microscopically. To determine the kinetics of cytosolic enzyme release from the cells, media samples were collected at various points of time and assayed for enzyme activity. To determine the cellular ATP, cells were washed with sodium phosphate buffer, scraped off and boiled for 3 min with sodium phosphate buffer. Following centrifugation the supernatant was collected and ATP was determined by the chemiluminogenic assay using firefly tails. The present results indicate that nitroxide stable free radicals, in the millimolar concentration range, provide full protection without toxic side-effect. Unlike exogenously added SOD that failed to protect, exogenous catalase provided almost full protection. In addition, the metal-chelating agent dipyridyl, but not diethylene-triamine-pentaacetate or desferrioxamine, protected the cultured cells. The present results suggest that H2O2 is the predominant toxic species mediating the oxidative damage whereas extracellular superoxide radical does not contribute to cultured cardiomyocyte damage. Since nitroxides do not remove H2O2 they can protect the cells possibly by oxidizing the metal ions and inhibiting the Fenton reaction. The superoxide dismutase-mimic activity of nitroxides does not seem to underlie their protective effect, however, the involvement of intracellular O .-2 cannot be excluded.
Similar content being viewed by others
Abbreviations
- CHDO:
-
2-spirocyclohexane doxyl (2-cyclohexane-5,5-demethyl-3-oxazolidinoxyl)
- DF:
-
desferrioxamine
- DTPA:
-
diethylene-triamine-pentaacetate
- EPR:
-
electron paramagnetic resonance
- HX:
-
hypoxanthine
- LDH:
-
lactate dehydrogenase
- SOD:
-
superoxide dismutase
- SEM:
-
standard error of mean: TEMPOL, 4-hydroxy-2,2,6,6-tetramethyl-piperidinoxyl
- TEMPAMINE:
-
4-amino-2,2,6,6-tetramethyl-piperidinoxyl
- XO:
-
xanthine oxidase
- CAT:
-
catalase
References
Bagchi M, Prasad MR, Engelman RM, Das DK: Effects of free radicals on the fluidity of myocardial membranes. Free Radical Res Commun 7: 375–380, 1989
Bolli R: Oxygen-derived free radicals and postischemic myocardial dysfunction (‘stunned myocardium’). J Am Cell Cardiol 12: 239–249, 1988
Laurindo FR, da Luz LP, Uint L, Rocha TF, Jaeger RG, Lopes EA: Evidence for superoxide radical-dependent coronary vasospasm after angioplasty in intact dogs. Circulation 83: 1705–1715, 1991
Engler R, Covell JW: Granulocytes cause reperfusion ventricular dysfunction after 15-min ischemia in the dog. Circ Res 61: 20–28, 1987
Esser E, Loschen G: Leukocytic O .-2 and cardiac dysfunction in isolated perfused rat hearts. Arch Toxicol 65: 361–365, 1991
Myers CL, Weiss SJ, Kirsh MM, Shlafer M: Involvement of hydrogen peroxide and hydroxyl radical in the ‘oxygen paradox’: reduction of creatine kinase release by catalase, allopurinol or deferoxamine, but not by superoxide dismutase. J Mol Cell Cardiol 17: 675–684, 1985
Zweier JL, Rayburn BK, Flaherty JT, Weisfeldt ML: Recombinant superoxide dismutase reduces oxygen free radical concentrations in reperfused myocardium. J Clin Invest 80: 1728–1734, 1987
Shlafer M, Kane PF, Kirsh MM: Superoxide dismutase plus catalase enhance the efficacy of hypothermic cardioplegia to protect the globally ischemic, reperfused heart. J Thorac Cardiovasc Surg 83: 830–839, 1982
Werns SE, Shea MJ, Driscoll EM, Cohen C, Abrams GD, Pitt B, Lucchesi BR: The independent effects of oxygen radical scavengers on canines infarct size. Reduction by superoxide dismutase but not catalase. Cir Res 56: 895–898, 1985
Chambers DJ, Braimbridge MV, Hearse DJ: Free radicals and cardioplegia. Free radical scavengers improve postischemic function of rat myocardium. Eur J Cardiothorac Surg 1: 37–45, 1987
Myers CL, Weiss SJ, Kirsh MM, Shepard BM, Shlafer M: Effects of supplementing hypothermic crystalloid cardioplegic solution with catalase, superoxide dismutase, allopurinol, or deferoxamine on functional recovery of globally ischemic and reperfused isolated hearts. J Thorac Cardiovasc Surg 91: 281–289, 1986
Berliner LJ: Spin labelling in enzymology: Spin-labeled enzymes and proteins. Methods Enzymol 49: 418–480, 1978
Brasch RC: Work in progress: methods of contrast enhancement for NMR imaging and potential applications. A subject review. Radiology 147: 781–788, 1983
Samuni A, Godinger D, Aronovitch J, Russo A, Mitchell JB: Nitroxides block DNA scission and protect cells from oxidative damage. Biochemistry 30: 555–561, 1991
Samuni A, Winkelsberg D, Pinson A, Hahn SM, Mitchell JB, Russo A: Nitroxide stable radicals protect beating cardiomyocytes against oxidative damage. J Clin Invest 87: 1526–1530, 1991
Gelvan D, Saltman P, Powell SR: Cardiac reperfusion damage prevented by a nitroxide free radical. Proc Natl Acad Sci USA 88: 4680–4684, 1991
Pinson A, Padieu P, Harari I: Techniques for culturing heart cells. In: A. Pinson (ed.). The heart cell in culture. CRC Press Inc., Boca Raton, 1987, pp 7–22
Wroblewski F, LaDue JS: Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med 90: 210–213, 1955
Holmsen H, Storm E, Day HJ: Determination of ATP and ADP in blood platelets-modification of firefly luciferase assay for plasma. Anal Biochem 46: 489–509, 1972
Beresewicz A, Horackova M: Alterations in electrical and contractile behavior of isolated cardiomyocytes by hydrogen peroxide: possible ionic mechanisms. J Mol Cell Cardiol 23: 899–918, 1991
Janero DR, Hreniuk D, Sharif HM: Hydrogen peroxide-induced oxidative stress to the mammalian heart-muscle cell (cardiomyocyte): lethal peroxidative membrane injury. J Cell Physiol 149: 347–364, 1991
Williams RE, Zweier JL, Flaherty JT: Treatment with deferoxamine during ischemia improves functional and metabolic recovery and reduces reperfusion-induced oxygen radical generation in rabbit hearts. Circulation 83: 1006–1014, 1991
Miki S, Ashraf M, Salka S, Sperelakis N: Myocardial Dysfunction and Ultrastructural Alterations Mediated by Oxygen Metabolites. J M Cell Cardiol 20: 1009–1024, 1988
Katoh S, Toyama J, Itsuo K, Toshiaki A, Abe T: Deferoxamine, an iron chelator, reduces myocardial injury and free radical generation in isolated neonatal rabbit hearts subjected to global ischemia-reperfusion. J Mol Cell Cardiol 24: 1267–1275, 1992
Ambrosio G, Zweier JL, Jacobus WE, Weisfeldt ML, Flaherty JT: Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: the role of iron in the pathogenesis of reperfusion injury. Circulation 76: 906–915, 1987
Samuni A, Krishna CM, Riesz P, Finkelstein E, Russo A: Superoxide reaction with nitroxide spin-adducts. Free Radical Biol Med 6: 141–148, 1989
Samuni A, Mitchell JB, DeGraff W, Krishna CM, Samuni U, Russo A: Nitroxide SOD-mimics: modes of action. Free Radical Res Commun 1: 187–194, 1991
Masarwa M, Cohen H, Meyerstein D, Hickman DL, Bakac A, Espenson JH: Reactions of low-valent transition-metal complexes with hydrogen peroxide. Are they ‘Fenton-like’ or not? The case of Cu+ and Cr2+. J Am Chem Soc 110: 4293–4297, 1988
Mitchell JB, Samuni A, Krishna MC, DeGraff WG, Ahn MS, Samuni U, Russo A: Biologically active metal-independent superoxide dismutase mimics. Biochemistry 29: 2802–2807, 1990
Robbins WK, Eastman RH: Photodecarbonylation in solution. II. Trapping of intermediates in the photolysis of dibenzyl ketone. J Am Chem Soc 92: 6077–6079, 1970
Chateauneuf J, Lusztyk J, Ingold K: Absolute rate constants for the reactions of some carbon-centered radicals with 2,2,6,6-tetramethylpiperidine-N-oxyl. J Organic Chemistry 53: 1629–1632, 1988
Nilsson UA, Bylund-Fellenius AC: Nitroxide compounds for the preparation of a pharmaceutical composition and method for the prophylaxis and treatment of ischemic cell damage. Patent Pending WO 8805044 AI 880714
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mohsen, M., Pinson, A., Zhang, R. et al. Do nitroxides protect cardiomyocytes from hydrogen peroxide or superoxide?. Mol Cell Biochem 145, 103–110 (1995). https://doi.org/10.1007/BF00935482
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00935482