Summary
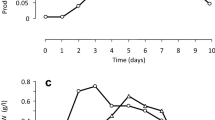
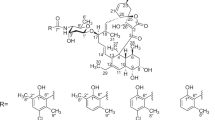
Two macrolide-like antibiotics were detected in the fermentation broth of an actinomycete (isolate 69B200) when grown either at 25 or 35°C. This strain showed high resistance to erythromycin, but was sensitive to non-macrolide antibiotics. The purified antibiotic from cultures grown at 25°C (compound I) showed activity againstEscherichia coli: compound II, produced at 35°C, was active againstStaphylococcus aureus. The molecular weights, determined by mass spectrometry, of compounds I and II are 293 and 307, respectively. Both compounds showed peaks in the u.v. spectra at 225 and 275 nm. Infra-red analysis indicated the presence of hydroxyl and carbonyl groups in both compounds: a lactone group was detected in compound II. The ‘H’-NMR spectrum demonstrated the presence of methyl groups in both compounds. Amino sugar is present in both compounds. The physico-chemical properties of the compounds do not correspond to those of any of the known macrolide antibiotics.
Résumé
Deux antibiotiques de type macrolide ont été décelés dans le bouillon de culture d'un actinomycète (souche 69B200). Cette souche présente une forte résistance à l'érythromycine, mais est sensible aux antibiotiques n'appartenant pas au groupe des macrolides. L'antibiotique purifié à partir des cultures développées à 25°C (composé I) est actif à l'égard deEscherichia coli; le composé II, produit à 35°C, agit surStaphylococcus aureus. Les poids moléculaires des composées I et II, déterminés par spectrographie de masse, sont respectivement de 293 et 307 daltons. Les spectres u.v. des deux composés présentent des pics à 225 et 275 nm. L'analyse dans l'i.r. révèle la présence de groupements hydroxyle et carbonyle dans les deux substances et un groupement lactone a été détecté dans le composé II. Le spectre RMH-H montre la présence de groupements méthyle dans les deux substances. D'autre part, chacune d'elles contient un sucre aminé. Les propriétés physico-chimiques de ces composés ne correspondent à celles d'aucun antibiotique macrolide antérieurement connu.
Resumen
Dos antibióticos de tipo macrólido fueron detectados en el caldo de fermentación de un actinomicete (aislado 69B200) cuando éste crecía tanto a 25°C como a 35°C. Esta cepa era muy resistente a la eritromicina siendo, sin embargo, sensible a antibióticos no macrólidos. El antibiótico purificado, a partir de cultivos que crecían a 25°C (compuesto I), mostró actividad contraE. coli. El compuesto II, producido a 35°C, era activo contraS. aureus. Los pesos moleculares, determinados mediante espectrometría de masas, de los compuestos I y II fueron, respectivamente, 293 y 307. Ambos compuestos mostraron picos en el espectro u.v. a 225 y 275 m. Los análisis mediante i.r. mostraron la presencia de grupos hidroxilo y carbonilo en ambos compuestos y de un grupo lactona en el compuesto II. El espectro RMN-‘H’ indicó la existencia de grupos metilo en ambos compuestos. En los dos compuestos hay un azúcar aminado. Las propeidades físico-químicas de ambos compuestos no se corresponden con las de ningún otro antibiótico macrólido conocido hasta el momento.
Similar content being viewed by others
References
Aharonowitz, Y. &Demain, A. L. 1978 Carbon catabolite regulation of cephalosporin production inStreptomyces clavuligerus.Antimicrobial Agents and Chemotherapy 14, 159–164.
Berdy, J., Aszalos, A., Bostain, M. &Mcnitt, K. L. 1980 Macrocyclic lactone (lactam) antibiotics. InHandbook of Antibiotic Compounds, Vol. 2. Boca Raton, Florida: CRC Press, Inc.
Buchanan, R. E. &Gibbons, N. E. 1974 Actinomycetales. InBergey's Manual of Determinative Bacteriology, 8th edn. pp. 750–827. Baltimore, U.S.A.: The Williams and Wilkins Co.
Ibrahim, M. A. K., Al-Diwany, L. J., Khayat, A. I., Kaddouri, N. F. &Kaddir, F. W. 1984 Antibacterial activities ofStreptomyces isolated from Mousel forest soil.Journal of Biological Science Research 15, 17–27.
Iwai, Y. &Omura, S. 1982 Culture conditions for screening of new antibiotics.Journal of Antibiotics 35, 123–141.
Majer, J., Martin, J. R., Egan, R. S. &Corcoran, J. W. 1977 Antibiotic glycosides. 8. erythromycin D, a new macrolide antibiotic.Journal of the American Chemical Society 99, 1620–1622.
Majer, J. 1978 Macrolide antibiotics. InAntibiotics, Isolation, Separation and Purification. Journal of Chromotography Library, Vol. 15, ed. Weinstein, M. J. & Wagman, G. H. pp. 273–308. Amsterdam: Elsevier Scientific Publishing Company.
Martin, J. R., Perun, T. J. &Girolami, R. L.1966 Studies on the biosynthesis of erythromycin. I. Isolation and structure of an intermediate glycoside, 3-a-L-mycarosylerythronolide B.Biochemistry 5, 2852–2856.
Oser, B. L. 1965Hawik's Physiological Chemistry 14th edn. New York: McGraw-Hill Book Co. Inc.
Prescott, S. C. &Dunn, C. G.1959 Antibiotics. InIndustrial Microbiology 3rd edn, pp. 762–853. New York: McGraw-Hill Book Co. Inc.
Shimizu, K-I. &Tamura, G. 1981 Reductionmycin, a new antibiotic I. Taxonomy, fermentation, isolation, characterization and biological activities.Journal of Antibiotics 34, 649–653.
Shirling, E. B. &Gottlieb, D. 1966 Methods of characterization ofStreptomyces species.International Journal of Systematic Bacteriology 16, 313–340.
Shomura, T., Kojima, M., Yoshida, J., Ito, M., Amano, S., Totsugawa, K., Niwa, T., Inouye, S., Ito, T. &Niida, T.1980 Studies on a new aminoglycoside antibiotic, dactimicin. I. Producing organism and fermentation.Journal of Antibiotics 33, 924–930.
Singh, S. K., Mandal, S. K. &Roy, D. K. 1981 Studies on erythromycin fermentation: nutritional requirement ofStreptomyces erythreus for erythromycin production.Indian Journal of Pharmaceutical Science 43, 137–141.
Spagnoli, R., Cappelletti, L. &Toscano, L.1983 Biological conversion erythronolide B, an intermediate at erythromycin biogenesis, into new “hybrid” macrolide antibiotics.Journal of Antibiotics 36, 365–375.
Yoshida, N., Tani, Y. &Ogata, K. 1972 Cryomycin, a new antibiotic produced only at low temperature.Journal of Antibiotics 25, 653–656.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hussain, H.A., Ibrahim, M.A.K., Mahmood, M.J. et al. Temperature-dependent production of two macrolide-like antibiotics produced by an actinomycete (isolate 69B200). Mircen Journal 2, 319–326 (1986). https://doi.org/10.1007/BF00933498
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00933498