Abstract
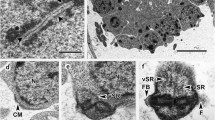
The in situ localization of antigenic substances inTrichinella spiralis muscle larvae was demonstrated at the subcellular level. Larvae recovered from mouse muscle were fixed with halfstrength Karnovsky fixative, dehydrated with alcohol, and embedded in LR White resin. Ultrathin sections were incubated with sera from infected Wistar rats and, subsequently, protein A-gold complex. The specificity of the immunostaining was confirmed by a control experiment. Positively immunostaining structures included the stichocyte granules, body cuticle, hindgut cuticle, hypodermis, hemolymph, glycogen aggregates, esophagusoccupying substance (EOS), midgut-occupying substance (MOS), brush border, cytoplasmic granules in the cord, intestinal gland cell granules, and discrete areas in the genital primordial cell. However, the esophageal cuticle, nucleus, nucleolus, mitochondria, rough endoplasmic reticulum, and muscle fibers were negative by immunostaining.
Similar content being viewed by others
References
Chitwood BG (1930) The structure of the esophagus in the Trichuroidea. J Parasitol 17: 35–42
Despommier DD (1974) The stichocyte ofTrichinella spiralis during morphogenesis in the small intestine of the rat. In: Kim C (ed) Trichinellosis. Intext Educational Publishers, New York, pp 239–254
Despommier DD (1977) Immunity toTrichinella spiralis. Am J Trop Med Hyg 26: 68–75
Despommier DD (1981) Partial purification and characterization of protection-inducing antigens from the muscle larva ofTrichinella spiralis by molecular sizing chromatography and preparative flatbed isoelectric focusing. Parasite Immunol 3: 261–272
Despommer DD (1983) Biology. In: Campbell WC (ed)Trichinella and trichinosis. Plenum Press New York London, pp 75–151
Despommier DD, Lacetti A (1981)Trichinella spiralis: proteins and antigens isolated from a large-particle fract on derived from the muscle larva. Exp Parasitol 51: 279–295
Furuki J, Takahashi Y, Uno T, Nishiyama T, Aisaka A, Yagi J, Araki T (1987) Morphological studies onTrichinella spiralis (4) with emphasis on the esophagus. J Electron Microsc 36: 308A
Geoghegan WD, Ackerman GA (1977) Adsorption of horseradish peroxidase, ovomucoid and antiimmunoglobulin to colloidal gold for the indirect detection of concanavalin A, wheat germ agglutinin and goat antihuman immunoglobulin G on cell surfaces at the electron microscopic level: a new method, theory and application. J Histochem Cytochem 25: 1187–1200
Jackson GJ (1959) Fluorescent antibody studies ofTrichinella spiralis infections. J Infect Dis 105: 97–117
Lee DL (1965) The cuticle of adultNippostronglus brasiliensis. Parasitology 55: 173–181
Ljungström I, Ruitenberg EJ (1976) A comparative study of the immunohistological and serological response of the intact and T-cell derived mouse toTrichinella spiralis Clin Exp Immunol 24: 146–156
Maizels RM, Philipp M, Ogilvie BM (1982) Molecules on the surface of parasitic nematodes as probes of the immune response in infection. Immunol Rev 61: 109–136
Mota I, Sadun E, Bradshaw RM, Gore RW (1969) The immunological response of mice infected withTrichinella spiralis. Immunology 16: 71–81
Oliver-Gonzales J (1940) The in vitro action of immune serum on the larvae and adults ofTrichinella spiralis. J Infect Dis 67: 292–300
Ottesen EA, Smith TK, Kirkpatrick CH (1975) Immune responses toTrichinella spiralis in the rat: I. Development of cellular and humoral responses. Int Arch Allergy Appl Immunol 49: 396–410
Richels I (1955) Histologische Studien zu den Problemen der Zellkonstanz: Untersuchungen zur mikroskopischen Anatomie im Lebenszyklus vonTrichinella spiralis. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt. Orig Reihe A 163: 46–84
Silberstein DS, Despommier DD (1984) Antigens fromTrichinella spiralis that induce a protective response in the mouse. J Immunol 132: 898–904
Silberstein DS, Despommier DD (1985a) Effects onTrichinella spiralis of host response to purified antigens. Science 227: 948–950
Silberstein DS, Despommier DD (1985b) Immunization with purifed antigens protects mice from lethal infection withTrichinella spiralis. J Parasitol 71:516–517
Slot JW, Geuze HJ (1985) A new method of preparing gold probes for multiple-labeling cytochemistry. Eur Cell Biol 38:87–93
Suzuki T, Sato Y, Yamashita T, Sekikawa H, Otsuru M (1974) Anisakiasis: preparation of a stable antigen for indirect fluorescent antibody test. Exp Parasitol 35:418–424
Takahashi Y, Yoshikawa Y, Furuki J, Yamada S, Araki T (1987) Morphological study ofTrichinella spiralis: an overall picture of a muscle larva as revealed by a longitudinal sectioning. Jpn J Parasitol 36:361–366
Takahashi Y, Uno T, Furuki J, Yamada S, Araki T (1988a) The morphology ofTrichinella spiralis: ultrastructural study of the mid- and hindgut of the muscle larvae. Parasitol Res 75:19–27
Takahashi Y, Uno T, Furuki J, Yamada S, Araki T (1988b) Morphology of the alimentary tract ofTrichinella spiralis muscle larva with emphasis on the esophagus. Parasitol Res 75:42–49
Taliaferro WH, Sarles MP (1939) The cellular reactions in the skin, lungs and intestine of normal and immune rats after infection withNippostrongylus muris. J Infect Dis 64:157–192
Tanaka H, Haga S, Takatsuki K, Yamaguchi K (1984) Localization of adult T-cell leukemia-associated antigens by the immunocolloidal gold method. Cancer Res 44:3493–3504
Villella JB (1970) Life cycle and morphology. In: Gould SE (ed) Trichinosis in man and animals. CC Thomas, Springfield, Illinois, pp 19–60
Wakelin D, Denham DA (1983) The immune response. In: Campbell WC (ed)Trichinella spiralis and trichinosis. Plenum Press, New York, pp 265–308
Wu LY (1955) The development of the stichosome and associated structures inTrichinella spiralis. Can J Zool 33:440–446
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takahashi, Y., Uno, T., Mizuno, N. et al. Ultrastructural localization of antigenic substances inTrichinella spiralis . Parasitol Res 75, 316–324 (1989). https://doi.org/10.1007/BF00931817
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00931817