Abstract
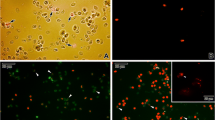
Eimeria tenella sporozoites exposed to 100, 70, 60 and 50 μg salinomycin sodium (SAL)/ml medium 199 at 41° C and then stained with propidium iodide/fluorescein diacetate were analysed by means of flow cytometry (FCM). After 20 min exposure, they showed dose-dependent alterations in their size and shape, i.e. ballooning of most cells, and enhanced intracellular esterase activity as compared with untreated controls. After longer exposure periods (40 and 70 min), inflated cells gradually changed into shrivelled or crumpled, nonviable ones, thereby showing a gradual decrease in esterase activity and a gradual loss of membrane integrity (RFA+). As compared with untreated controls, sporozoites treated with 10 μg SAL/ml showed negligible RFA+ values (0.4%–2%), whereas those exposed to 1 and 0.1 μg SAL/ml and to the solvent dimethylsulfoxide (DMSO, 1%) did not, even after 70 min exposure. Slight to severe structural changes manifesting as an extremely wavy surface (1 μg SAL/ml), vacuolization of the cytoplasm, distension or destruction of the mitochondrion and rupture of cell membranes (10 μg SAL/ml) were seen not only at higher SAL concentrations but also (rarely) at lower ones. The ability of sporozoites to invade primary chick-kidney cells was significantly inhibited by 70, 60 and 50 μg SAL/ml. In general, there were close relationships between findings obtained using FCM, electron microscopy and an invasion-inhibition test. The results indicate that FCM is a reliable and sensitive technique for characterizing the parasiticidal effects on and the possible mode of action of drugs in free coccidian sporozoites.
Similar content being viewed by others
References
Britten V, Murphy J (1986) Cell sorting by continuous flow cytometry. Parasitol Today 2:85–87
Chappel LH (1988) The interactions between drugs and the parasite surface. Parasitology 96:S167-S193
Coutinho SG, Louis JA, Mauel J, Engers HD (1984) Induction by specific T lymphocytes of intracellular destruction ofLeishmania major in infected murine macrophages. Parasite Immunol 6:157–170
Dallas CE, Evans DL (1990) Flow cytometry in toxicity analysis. Nature 345:557–558
Ehrlich K, Hofmann J, Raether W, Bräu B (1990) Durchflußzytophotometrische Untersuchungen zur Wirkungsweise von Salinomycin-Natrium aufEimeria tenella Sporozoiten (abstract). Proceedings, 3. Heidelberger Flow-Zytometrie Symposium, Heidelberg, Oktober 18–20
Franklin RM, Brun R, Grieder A (1986) Microscopic and flow cytophotometric analysis of parasitemia in cultures ofPlasmodium falciparum vitally stained with Hoechst 33342 — application to study of antimalarial agents. Z Parasitenkd 72:201–212
Fuller AL, McDougald LR (1989) Analysis of coccidian oocyst populations by means of flow cytometry. J Protozool 36:143–146
Hare JD, Bahler DW (1986) Analysis ofPlasmodium falciparum growth in culture using acridine orange and flow cytometry. J Histochem Cytochem 34:215–220
Hofmann J, Raether W (1985) Modified PAS-AO techniques for detecting early endogenous developing stages ofEimeria sp. (abstract). Proceedings 5th Japanese-German Cooperative Symposium on Protozoan Diseases, Tokyo, September 25–27
Hofmann J, Raether W (1990) Improved techniques for the in vitro cultivation ofEimeria tenella in primary chick kidney cells. Parasitology 76:479–486
Hoyne GF, Boreham PFL, Parsons PG, Ward C, Biggs B (1989) The effect of drugs on the cell cycle ofGiardia intestinalis. Parasitology 99:333–339
Janse CJ, Vianen PH van, Tanke HJ, Mons B, Ponnudurai T, Overdulve JP (1987)Plasmodium species: flow cytometry and microfluorometry assessment of DNA content and synthesis. Exp Parasitol 64:88–94
Lehninger AL (1981) Biochemistry, 2nd edn. Worth, New York
Libertin CR, Woloschak GE, Wilson WR, Smith TF (1984) Analysis ofPneumocystis carinii cysts with a fluorescence-activated cell sorter. Clin Microbiol 20:877–880
Luft BJ, Kansas G, Engleman EG, Remington JS (1984) Functional and quantitative alterations in T lymphocyte subpopulations in acute toxoplasmosis. J Infect Dis 150:761–767
Mehlhorn H (1988) Parasitology in focus. Springer, Berlin Heidelberg New York Tokyo
Mehlhorn H, Pooch H, Raether W (1983) The action of polyether ionophorous antibiotics (monensin, salinomycin, lasalocid) on the development stages ofEimeria tenella (coccidia, sporozoa) in vivo and in vitro: study by light and electron microscopy. Z Parasitenkd 69:457–471
Melamed MR, Mullancy PF, Mendelsohn ML (1979) Flow cytometry and sorting. Wiley, New York
Pressman BC, Harris EJ, Jagger WS, Johnson JH (1967) Antibiotic mediated transport of alkali ions across lipid barriers. Proc Natl Acad Sci USA 58:1949–1956
Raether W (1988) Control of the coccidia proper in live-stock and poultry. In: Mehlhorn H (ed) Parasitology in focus. Springer, Berlin Heidelberg New York Tokyo, pp 741–755
Raether W, Hofmann J, Bräu B, Ehrlich K, Hammann P (1990) Cytofluorometric studies on salinomycin treatedE. tenella sporozoites (abstract). Proceedings, VII International Congress on Parasitology, Paris, August 20–24
Shapiro HM (1985) Parameters and probes In: Shapiro HM (ed) Practical flow cytometry. Alan J Liss, New York, pp 84–158
Smith CK, Galloway RB (1983) Influence of monensin on cation influx and glycolysis ofEimeria tenella sporozoites in vitro. J Parasitol 69:666–670
Smith CK, Strout RG (1980)Eimeria tenella: effect of narasin, a polyether antibiotic on the ultrastructure of intracellular sporozoites. Exp Parasitol 50:426–436
Smith ChK II, Galloway RB, White SL (1981) Effect of ionophores on survival, penetration and development ofEimeria tenella sporozoites in vitro. J Parasitol 67:511–516
Thomson JE, Fernando MA, Pasternak J (1979) Induction of gelphase lipid in plasma membrane of chick intestinal cell after coccidial infection. Biochim Biophys Acta 555:472–484
Uemura T, Suzuki S, Ohnishi T (1990) Flow cytometric enumeration of reticulocyte in the peripheral blood from canine infected withBabesia gibsoni. J Vet Med [B] 37:468–472
Wang CC (1982) Biochemistry and physiology of coccidia. In: Long PL (ed) The biology of the coccidia. Arnold, London, pp 167–228
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raether, W., Mehlhorn, H., Hofmann, J. et al. Flow cytometric analysis ofEimeria tenella sporozoite populations exposed to salinomycin sodium in vitro: A comparative study using light and electron microscopy and an in vitro sporozoite invasion-inhibition test. Parasitol Res 77, 386–394 (1991). https://doi.org/10.1007/BF00931633
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00931633