Abstract
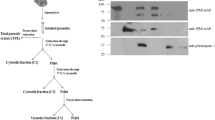
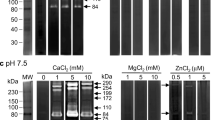
An endopeptidase specific to thePlasmodium falciparum erythrocytic schizont stage and to free merozoites was detected using the fluorogenic GlcA-Val-Leu-Gly-Lys(or Arg)-AEC substrate. The enzyme was purified by high performance liquid chromatography (HPLC); its optimal activity was around pH 7.5 and its isoelectric point was 4.4. The molecular weight of the enzyme was about 68000, as demonstrated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The endopeptidase was strongly inhibited by thiol proteinase inhibitors, leupeptin, and antipain. The possible involvement of this neutral endopeptidase in the reinvasion process is discussed.
Similar content being viewed by others
Abbreviations
- GlcA:
-
gluconoyl group
- AEC:
-
3-amino-9-ethylcarbazole
- EDTA:
-
ethylenediaminetetraacetic acid
- PHMS:
-
p-hydroxymercuriphenylsulfonic acid
- PMSF:
-
phenylmethylsulfonyl fluoride
- STI:
-
soybean trypsin inhibitor
- DMEM:
-
Dulbecco's modified Eagle's medium
- HEPES:
-
4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid
- PBS:
-
phosphate-buffered saline
- SDS-PAGE:
-
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- ID:
-
inhibitory dose
- bis-TRIS:
-
[bis(2-hydroxyethyl)imino-tris(hydroxymethyl)methane]
References
Aikawa M, Miller LH, Johnson J, Rabbege J (1979) Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol 77:72–82
Banyal HS, Misra GC, Gupta CM, Dutta GP (1981) Involvement of malarial proteases on the interaction between the parasite and host erythrocyte inPlasmodium knowlesi infections. J Parasitol 67:623–626
Bernard F, Schrével J (1987) Purification of aPlasmodium berghei neutral endopeptidase and its localization in merozoite. Mol Biochem Parasitol 26:167–174
Bernard F, Mayer R, Picard I, Deguercy A, Monsigny M, Schrével J (1987)Plasmodium berghei andPlasmodium chabaudi: a neutral endopeptidase in parasite extracts and plasma of infected animals. Exp Parasitol 64:95–103
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
David PH, Hadley TJ, Aikawa M, Miller LH (1984) Processing of a major parasite surface glycoprotein during the ultimate stages of differentiation inPlasmodium knowlesi. Mol Biochem Parasitol 11:267–282
Dejkriengkraikhul P, Wilairat P (1983) Requirement of malarial protease in the invasion of human red cells by merozoites ofPlasmodium falciparum. Z Parasitenkd 69:313–317
Dluzewski AR, Rangachari K, Wilson RJM, Gratzer WB (1986)Plasmodium falciparum: protease inhibitors and inhibition of erythrocyte invasion. Exp Parasitol 62:416–422
Hadley TJ, Aikawa M, Miller LH (1983)Plasmodium knowlesi: studies in invasion of rhesus erythrocytes by merozoites in the presence of protease inhibitors. Exp Parasitol 55:306–311
Hall R, Osland A, Hyde JE, Simmons DL, Hope IA, Scaife JG (1984) Processing, polymorphism and biological signification of P190, a major surface antigen of the erythrocytic forms ofPlasmodium falciparum. Mol Biochem Parasitol 11:61–80
Heussen C, Dowdle EB (1980) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem 102:196–202
Holder AA, Freeman RR (1984) The three major antigens on the surface ofPlasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med 160:624–629
Inselburg J, Banyal H (1984)Plasmodium falciparum: synchronization of asexual development with aphicicolin, a DNA synthesis inhibitor. Exp Parasitol 57:48–54
Johnson JG, Epstein N, Shiroishi T, Miller LH (1981) Identification of surface proteins of viablePlasmodium knowlesi merozoites. J Protozool 28:160–169
Ladda R, Aikawa M, Sprinz H (1969) Penetration of erythrocytes by merozoites of mammalian and avian malarial parasites. J Parasitol 55:633–644
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lambros C, Vanderberg JP (1979) Synchronization ofPlasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420
Lyon JA, Haynes JD (1986)Plasmodium falciparum antigens synthesized by schizonts and stabilized at the merozoite surface when schizonts mature in the presence of protease inhibitors. J Immunol 136:2245–2251
Merril CR, Goldman D, Sedman SA, Ebert MH (1981) Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science 211:1437–1438
Monsigny M, Mayer R (1983) Nouveaux peptides hydrosolubles:N-polyhydroxyalcanoyl peptides. French patent CNRS-ANVAR 83-08051
Monsigny M, Kiéda C, Maillet T (1982) Assay for proteolytic activity using a new fluorogenic substrate (peptidyl-3-amino-9-ethylcarbazole); quantitative determination of lipopolysaccharide at the level of one picogram. EMBO J 1:303–306
Mrema JEK, Langreth SG, Jost RC, Rieckmann KM, Heidrich HG (1982)Plasmodium falciparum: isolation and purification of spontaneously released merozoites by nylon membrane sieves. Exp Parasitol 54:285–295
Obrénovitch A, Maintier C, Maillet T, Mayer R, Kiéda C, Monsigny M (1983) Sensitive fluorometric determination of plasminogen activator in cell lysates and supernatants. FEBS Lett 197:265–270
Okoye VCN, Bennett V (1985)Plasmodium falciparum malaria: band 3 as a possible receptor during invasion of human erythrocytes. Science 227:169–171
Pasvol G, Wainscoat JS, Weatherall DJ (1982) Erythrocytes deficient in glycophorin resist invasion by the malarial parasitePlasmodium falciparum. Nature 297:64–65
Pirson PJ, Perkins ME (1985) Characterization with monoclonal antibodies of a surface antigen ofPlasmodium falciparum merozoites. J Immunol 134:1946–1951
Rosenthal PJ, Kim K, McKerrow JH, Leech JH (1987) Identification of three stage-specific proteinases ofPlasmodium falciparum. J Exp Med 166:816–821
Schrével J, Bernard F, Maintier C, Mayer R, Monsigny M (1984) Detection and characterization of a selective endopeptidase fromPlasmodium berghei by using fluorescent peptidyl substrates. Biochem Biophys Res Commun 124:703–710
Schrével J, Philippe M, Bernard F, Monsigny M (1986) SurfacePlasmodium sugar-binding components evidenced by fluorescent neoglycoproteins. Biol Cell 56:49–55
Trager W, Jensen JB (1976) Human malarial parasites in continuous culture. Science 193:673–675
Zolg JW, McLeod AJ, Dickson IH, Scaife JG (1982)Plasmodium falciparum: modifications of the in vitro culture conditions improving parasitic yields. J Parasitol 68:1072–1080
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grellier, P., Picard, I., Bernard, F. et al. Purification and identification of a neutral endopeptidase inPlasmodium falciparum schizonts and merozoites. Parasitol Res 75, 455–460 (1989). https://doi.org/10.1007/BF00930972
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00930972