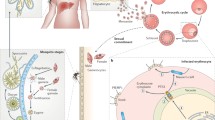
Abstract
On infecting the red blood cell, the malaria parasitePlasmodium falciparum induces alterations in the erythrocyte membrane. The parasite appears to synthesize proteins that are exported across the parasitophorous vacuole membrane and through a system of membraneous structures within the cytoplasm of the host cell to the surface membrane of the erythrocyte. There, these proteins are either released or remain associated with the membrane. In this review we describe the structure and discuss the possible functions of some of the exported proteins.
Similar content being viewed by others
References
Aikawa M, Uni Y, Andrutis A, Howard R (1986) Membrane-associated electron-dense material of the asexual stages ofPlasmodium falciparum: evidence for movement from the intracellular parasite to the erythrocyte membrane. Am J Trop Med Hyg 35:30–36
Allred DR, Gruenberg JE, Sherman IW (1986) Dynamic rearrangements of erythrocyte membrane internal architecture induced by infection withPlasmodium falciparum. J Cell Sci 81:1–16
Anderson RA, Marchesi VT (1985) Regulation of the association of membrane skeletal protein 4.1 with glycophorin by a polyphosphoinositide. Nature 318:295–298
Berendt AR, Ferguson DJP, Newbold CI (1990) Sequestration inPlasmodium falciparum malaria: sticky cells and sticky problems. Parasitol Today 6:247–254
Biggs BA, Gooze L, Wycherley K, Wilkinson D, Boyd AW, Forsyth KP, Edelman L, Brown GV, Leech JH (1990) Knob-independent cytoadherence ofPlasmodium falciparum to the leukocyte differentiation antigen CD36. J Exp Med 171:1883–1892
Brown GV, Culvenor J, Crewther P, Bianco A, Coppel R, Saint R, Stahl H, Kemp D, Anders R (1985) Localization of the ring-infected erythrocyte surface antigen (RESA) ofPlasmodium falciparum in merozoites and ring-infected erythrocytes. J Exp Med 162:774–779
Cappai R, Schravendijk MR van, Anders RF, Peterson MG, Thomas LM, Cowmann AF, Kemp DJ (1989) Expression of the RESA gene inPlasmodium falciparum isolate FCR3 is prevented by a subtelomeric deletion. Mol Cell Biol 9:3584–3587
Carlson J, Homquist G, Taylor DW, Perlmann P, Wahlgren M (1990) Antibodies to a histidine-rich protein (PfHRP1) disrupt spontaneously formedPlasmodium falciparum erythrocyte rosettes. Proc Natl Acad Sci USA 87:2511–2515
Coppel RL, Culvenor JG, Bianco AE, Crewther PE, Stahl H-D, Brown GV, Anders RF, Kemp DJ (1986) Variable antigen associated with the surface of erythrocytes infected with mature stages ofPlasmodium falciparum. Mol Biochem Parasitol 20:265–277
Favaloro JM, Coppel RL, Corcoran LM, Foote SJ, Brown GV, Anders RF, Kemp DJ (1986) Structure of the RESA gene ofPlasmodium falciparum. Nucleic Acids Res 14:8265–8277
Foley M, Murray LJ, Anders RF (1990) The ring-infected erythrocyte surface antigen protein ofPlasmodium falciparum is phosphorylated upon association with the host cell membrane. Mol Biochem Parasitol 38:69–76
Haldar K, Henderson CL, Cross G (1986) Identification of the parasite transferrin receptor ofPlasmodium falciparum-infected erythrocytes and its acylation via 1, 2-diacyl-sn-glycerol. Proc Natl Acad Sci USA 83:8565–8569
Howard RJ, Uni S, Aikawa M, Aley S, Leech J, Lew A, Wellems T, Rener J, Taylor D (1986) Secretion of a malarial histidinerich protein (PfHRP2) fromPlasmodium falciparum-infected erythrocytes. J Cell Biol 103:1269–1277
Howard RJ, Barnwell J, Rock E, Neequaye J, Ofori-Adjei D, Lee Maloy W, Lyon J, Saul A (1988) Two approximately 300 kilodaltonPlasmodium falciparum proteins at the surface membrane of infected erythrocytes. Mol Biochem Parasitol 27:207–224
Knapp B, Shaw A, Hundt E, Enders B, Küpper H (1988) A histidine alanine rich recombinant antigen protects Aotus monkeys fromP. falciparum infection. Behring Res Commun 82:349–359
Knapp B, Hundt E, Küpper HA (1989) A new blood stage antigen ofPlasmodium falciparum transported to the erythrocyte surface. Mol Biochem Parasitol 37:47–56
Koenen M, Scherf A, Mercereau O, Langsley G, Sibilli L, Dubois P, Pereira da Silva L, Müller-Hill B (1984) Human antisera detect aPlasmodium falciparum genomic clone encoding a nonapeptide repeat. Nature 311:382–385
Leech JH, Barnwell JW, Aikawa M, Miller LH, Howard RJ (1984a)Plasmodium falciparum malaria: association of knobs on the surface of infected erythrocytes with a histidine-rich protein and the erythrocyte skeleton. J Cell Biol 98:1256–1264
Leech JH, Barnwell JW, Miller LH, Howard RJ (1984b) Identification of a strain-specific malarial antigen exposed on the surface ofPlasmodium falciparum-infected erythrocytes. J Exp Med 159:1567–1575
Lustigman S, Anders RF, Brown GV, Coppel RL (1990) The mature-parasite-infected erythrocyte surface antigen (MESA) ofPlasmodium falciparum associates with the erythrocyte membrane skeletal protein, band 4.1. Mol Biochem Parasitol 38:261–270
Magowan C, Wollish W, Anderson L, Leech JH (1988) Cytoadherence byPlasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on the surface of infected erythrocytes. J Exp Med 168:1307–1320
Marsh K, Howard RJ (1986) Antigens induced on erythrocytes byP. falciparum: expression of diverse and conserved determinants. Science 231:150–153
Marsh K, Sherwood JA, Howard RJ (1986) Parasite-infected-cell-agglutination and indirect immunofluorescence assays for detection of human serum antibodies bound to antigens onPlasmodium falciparum-infected erythrocytes. J Immunol Methods 91:107–115
Mattei D, Berzins K, Wahlgren M, Udomsangpetch R, Perlmann P, Griesser HW, Scherf A, Müller-Hill B, Bonnefoy S, Guiltotte M, Langsley G, Pereira da Silva L, Mercereau-Puijalon O (1989) Cross-reactive antigenic determinants present on differentPlasmodium falciparum blood-stage antigens. Parasite Immunol 11:15–30
Panton LJ, McPhie P, Maloy L, Wellems T, Taylor D, Howard R (1989) Purification and partial characterization of an unusual protein ofPlasmodium falciparum: histidine-rich protein II. Mol Biochem Parasitol 35:149–160
Peterson C, Nelson R, Magowan C, Wollish W, Jensen J, Leech J (1989) The mature erythrocyte surface antigen ofPlasmodium falciparum is not required for knobs or cytoadherence. Mol Biochem Parasitol 36:61–66
Petersen C, Nelson R, Leech J, Jensen J, Wollish W, Scherf A (1990) The gene product of thePlasmodium falciparum 11.1 locus is a protein larger than one megadalton. Mol Biochem Parasitol 42:184–196
Pologe LG, Ravetch JV (1986) A chromosomal rearrangement in aP. falciparum histidine-rich protein gene is associated with the knobless phenotype. Nature 322:474–477
Pologe LG, Pavlovec A, Shio H, Ravetch JV (1987) Primary structure and subcellular localization of the knob-associated histidine-rich protein ofPlasmodium falciparum. Proc Natl Acad Sci USA 84:7139–7143
Ragge K, Arnold H, Tümmler M, Knapp B, Hundt E, Lingelbach K (1990) In vitro biosynthesis and membrane translocation of the serine rich protein ofPlasmodium falciparum. Mol Biochem Parasitol 41:93–100
Rock EP, Marsh K, Saul AJ, Wellems TE, Taylor DW, Maloy WL, Howard RJ (1987) Comparative analysis of thePlasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology 95:209–227
Rodriguez MH, Jungery M (1986) A protein onPlasmodium falciparum-infected erythrocytes functions as a transferrin receptor. Nature 324:388–391
Scherf A, Hilbich C, Sieg K, Mattei D, Mercereau-Puijalon O, Müller-Hill B (1988) The 11-1 gene ofPlasmodium falciparum codes for distinct fast evolving repeats. EMBO J 7:1129–1137
Sherman I, Winograd E (1990) Antigens on thePlasmodium falciparum infected erythrocyte surface are not parasite derived. Parasitol Today 6:317–320
Sherwood JA, Marsh K, Howard RJ, Barnwell JW (1985) Antibody mediated strain-specific agglutination ofPlasmodium falciparum-parasitized erythrocytes visualized by ethidium bromide staining. Parasite Immunol 7:659–663
Stanley HA, Reese RT (1986)Plasmodium falciparum polypeptides associated with the infected erythrocyte plasma membrane. Proc Natl Acad Sci USA 83:6093–6097
Stanley HA, Langreth SG, Reese RT (1989)Plasmodium falciparum antigens associated with membrane structures in the host erythrocyte cytoplasm. Mol Biochem Parasitol 36:139–150
Tanabe K (1990) Glucose transport in malaria infected erythrocytes. Parasitol Today 6:225–229
Taylor DW, Parra M, Chapman GB, Stearns ME, Rener J, Aikawa M, Uni S, Aley SB, Panton LJ, Howard RJ (1987) Localization ofPlasmodium falciparum histidine-rich protein 1 in the erythrocyte skeleton under knobs. Mol Biochem Parasitol 25:165–174
Triglia T, Stahl H, Crewther P, Scanlon D, Brown G, Anders R, Kemp D (1987) The complete sequence of the gene for the knob-associated histidine-rich protein fromPlasmodium falciparum. EMBO J 6:1413–1419
Udomsangpetch R, Aikawa M, Berzins K, Wahlgren M, Perlmann P (1989a) Cytoadherence of knoblessPlasmodium falciparum-infected erythrocytes and its inhibition by a human monoclonal antibody. Nature 338:763–765
Udomsangpetch R, Carlsson J, Wahlin B, Holmquist G, Ozaki LS, Scherf A, Mattei D, Mercereau-Puijalon O, Uni S, Aikawa M, Berzins K, Perlmann P (1989b) Reactivity of the human monoclonal antibody 33G2 with repeated sequences of three distinctPlasmodium falciparum antigens. J Immunol 142:3620–3626
Wellems TE, Howard RJ (1986) Homologous genes encode two distinct histidine-rich proteins in a cloned isolate ofPlasmodium falciparum. Proc Natl Acad Sci USA 83:6065–6069
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. Eckert (Zürich) on the occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Knapp, B., Hundt, E. & Lingelbach, K.R. Structure and possible function ofPlasmodium falciparum proteins exported to the erythrocyte membrane. Parasitol Res 77, 277–282 (1991). https://doi.org/10.1007/BF00930901
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00930901