Abstract
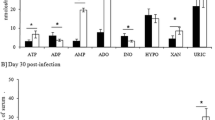
The stable metabolites of thromboxane A2, prostaglandin E2, and prostaglandin F2α (TxB2, PgEM, and PgFM, respectively) were measured in the blood plasma of nine castrated male pigs, each inoculated with 2×105 sporocysts ofSarcocystis miescheriana (group A), and in that of nine non-infected controls (group B). all infected pigs developed mild disease, the clinical signs being most severe between days 14 and 17 post infection (p.i.). In the infected pigs of group A, the TxB2 plasma levels increased with the onset of the acute phase of illness (12 day p.i), reaching peak values at day 14 p.i. The mean TxB2 values were significantly higher in the infected pigs from day 12 p.i. until the termination of the experiment on day 21 p.i. The PgEM values increased steadily in the infected pigs from day 12 p.i. until day 21 p.i. but remained relatively constant in the control pigs during the same period. In contrast, PgFM values remained low in the infected pigs throughout the experiment, and no significant differences between infected and non-infected pigs could be found. We conclude that the elevated TxB2 and PgEM values reflect a major involvement of prostanoids in the pathogenesis of sarcocystiosis.
Similar content being viewed by others
References
Baetz AL, Leiting SE, Bryner J, Barnett D (1981) Prepartum changes of plasma concentrations of prostaglandin F and 13,14-dihydro-15-ketoprostaglandin metabolites in pregnant animals exposed toSarcocystis cruzi orCampylobacter fetus. Am J Vet Res 42:22–24
Clark IA, Hunt NH (1986) Increased production of arachidonate metabolites by peritoneal cells of mice infected withPlasmodium vinckei vinckei. Aust J Exp Biol Med Sci 64:415–418
Creutzig A (1986) Prostaglandine und Gefäßerkrankungen. Med Klin 81:395–399
Das UN, Padma MC (1977) Presence of prostaglandin E and A inEntamoeba histolytica. Indian J Exp Biol 15:1227–1228
Daugschies A, Altfeld E, Rommel M (1989) Hemostatic alterations in pigs fed sublethal doses ofSarcocystis miescheriana. Vet Parasitol (in print)
Dubey JP, Speer CA, Fayer R (1989) Sarcocystosis of animals and man. CRC Press, Boca Raton
Edquist LE, Kindahl H, Stabenfeldt G (1978) Release of prostaglandin F2α during the bovine peripartal period. Prostaglandins 16:111–119
Essien EM, Arnout J, Deckmyn H, Vermylen J, Verstraete M (1984) Blood changes and enhanced thromboxane and 6-keto-prostaglandin F1α production in experimental acutePlasmodium berghei infection in hamsters. Thromb Haemostas 51:362–365
Fierer J, Salmon JA, Askonas BA (1984) African trypanosomiasis alters prostaglandin production by murine peritoneal macrophages. Clin Exp Immunol 58:548–556
Gambarini ML (1989) Untersuchungen zum Gerinnungsstatus und zur Gesamtöstrogen-sowie Prostaglandinkonzentration im peripartalen Zeitraum beim Rind. Thesis, School of Veterinary Medicine Hannover
Hadas E (1988) Biosynthesis of prostaglandins in pathogenic and non-pathogenic strains ofAcanthamoeba castellanii. Acta Protozool 27:61–66
Higgins AJ (1985) The biology, pathophysiology and control of eicosanoids in inflammation. J Vet Pharmacol Ther 8:1–18
Leid RW, McConnell LA (1983a) Thromboxane A2 generation by larval cestode,Taenia taeniaeformis. Clin Immunol Immunopathol 28:67–76
Leid RW, McConnell LA (1983b) PGE2 generation and release by the larval stage of the cestode,Taenia taeniaeformis. Prostagl Leukotr Med 11:317–323
McDaniel HT, Prestwood AK, Puette J, Dorough K, Strohlein DA (1983) Treatment of sarcosporidiosis in growing swine. Proc Annu Meet Assoc Swine Pract, Cincinnati, Oblo 17–19 April 1983:159
Reiner NE, Schultz LA, Malemud CJ (1988) Eicosanoid matabolism byLeishmania donovani-infected macrophages: mouse strain responses in prostanoid synthesis. Am J Trop Med Hyg 38:59–64
Ruebush MJ, Steel LK, Kennedy DA (1986) II. Prostaglandinmediated suppression of delayed-type hypersensitivity to infected erythrocytes duringBabesia microti infection in mice. Cell Immunol 98:300–310
Salafsky B, Fusco A (1987a) Eicosanoids as immunomodulators of penetration by schistosome cercariae. Parasitol Today 3:279–281
Salafsky B, Fusco AC (1987b)Schistosoma mansoni: a comparison of secreted vs nonsecreted eicosanoids in developing schistosomulae and adults. Exp Parasitol 64:361–367
Strohlein DA (1986) Contributions to the biology of experimentalSarcocystis suicanis infection in domestic swine. PhD Thesis, University of Georgia College of Veterinary Medicine
Torp C (1979) Untersuchungen über den Einfluß derSarcocystis muris-Infektion auf die Trächtigkeit und das Aufzuchtergebnis von NMRI-Mäusen. Thesis, School of Veterinary Medicine Hannover
Williams TJ, Peck MJ (1977) Role of prostaglandin-mediated vasodilation in inflammation. Nature 270:530–532
Author information
Authors and Affiliations
Additional information
Supported by the German Research Council (DFG)
Rights and permissions
About this article
Cite this article
Daugschies, A., Rommel, M. & Hoppen, H.O. Prostanoids during acute sarcocystiosis in growing pigs. Parasitol Res 76, 115–118 (1989). https://doi.org/10.1007/BF00930831
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00930831