Abstract
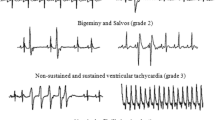
Although many causal factors have been proposed for the ischemia-reperfusion injury, the exact mechanisms for interdependent derangements of mechanical, electrical and metabolic events remains unclear. For this purpose, the Langendorff-perfused rat hearts were subjected to regional brief ischemia followed by reperfusion to study the protective effects of amiloride, an inhibitor of Na+−H+ exchange. Amiloride (0.1 mM) attenuated the rise in tissue Na+ and Ca2+, both duration and incidence of arrhythmias (p<0.05 vs. control), sarcolemmal injury (assessed by Na−K ATPase) and lipid peroxidation (assessed by malonedialdehyde formation) during reperfusion. Treatment of hearts with monensin, a sodium inophore, reversed the protective effects of amiloride. Reduction in transsarcolemmal Na+ and pH gradients during ischemia exhibited protective effects similar to those seen with amiloride. These results suggest that cardiac dysfunction, sarcolemmal injury and triggered arrhythmias during ischemia-reperfusion are due to the occurrence of intracellular Ca2+ overload caused by the activation of Na+−H+ exchange and Na+−Ca2+ exchange systems in the myocardium.
Similar content being viewed by others
References
Lazdunzki M, Frelin C, Vigne P: The sodium/hydrogen exchange system in cardiac cells: Its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol 17:1029–1042, 1985
Dennis SC, Coetzee WA, Cragoe EJ Jr, Opie LH: Effects of proton buffering and amiloride derivatives on reperfusion arrhythmias in isolated rat hearts: Possible evidence for an arrhythmogenic role of Na−H exchange. Circ Res 66:1156–1159, 1990
Coffe SM, Poole-Wilson PA: The time of onset and severity of acidosis in myocardial ischemia. J Mol Cell Cardiol 12:745–760, 1980
Pike MM, Kitakaze M, Marban E:23Na-NMR measurements of intracellular sodium in intact perfused ferret hearts during ischemia and reperfusion. Am J Physiol 259:H1767-H1773, 1990
Shen AC, Jennings RB: Kinetics of calcium accumulation in acute myocardial injury. Am J Pathol 67:441–452, 1972
Nayler WG, Poole-Wilson PA, Williams A: Hypoxia and calcium. J Mol Cell Cardiol 11:683–706, 1979
Nayler WG: The role of calcium in the ischemic myocardium. Am J Pathol 102:262–270, 1981
Steenbergen C, Murphy E, Levy L, London RE: Elevation of cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res 60:700–707, 1987
Philipson KD, Bersohn MM, Nishimoto AY: Effects of pH on Na−Ca exchange in canine cardiac sarcolemmal vesicles. Circ Res 50: 287–293, 1982
Tani M, Neely JR: Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts: Possible involvement of H+−Na+ and Na+−Ca2+ exchange. Circ Res 65:1045–1056, 1989
Kusuoka H, Porterfield JK, Weisman HF, Weisfeldt ML, Marban E: Pathophysiology and pathogenesis of stunned myocardium: Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest 79:950–961, 1987
du Toit EF, Opie LH: Modulation of severity of reperfusion stunning in the isolated rat heart by agents altering calcium flux at onset of reperfusion. Circ Res 70:960–967, 1992
Kloner RA, Ellis SG, Lange R, Braunwald E: Studies of experimental coronary artery reperfusion: Effects of infarct size, myocardial function, biochemistry, ultrastructure and microvascular damage. Circulation 68:I8-I15, 1983
Jennings RB, Schaper J, Hill ML, Steenbergen C Jr, Reimer KA: Effect of reperfusion late in the phase of reversible ischemic injury: Changes in cell volume, electrolytes, metabolites and ultrastructure. Circ Res 56:262–278, 1985
Hearse DJ, Tosaki A: Free radicals and calcium: Simultaneous interacting triggers as determinants of vulnerability to reperfusion-induced arrhythmias in the rat heart. J Mol Cell Cardiol 20:213–223, 1988
Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, Cobb SM, Coker SJ, Harness JB, Harron DWG, Higgins AJ, Julian DG, Lab MJ, Manning AS, Northover BJ, Parratt JR, Riemersma RA, Riva E, Russel DC, Sheridan DJ, Winslow E, Woodward B: Guidelines for the study of arrhythmias ischemia, infarction and reperfusion. Cardiovasc Res 22:447–455, 1988
Brittain-Valenti K, Danilo P Jr, Rosen MR: Modulation of reperfusion-induced arrhythmias by α1-adrenergic receptor subtypes in the cat (Abstr). Circulation (suppl) 84:II-494, 1991
Alto LE, Dhalla NS: Myocardial cation contents during induction of calcium paradox. Am J Physiol 237:H713-H719, 1979
Makino N, Dhalla KS, Elimban V, Dhalla NS: Sarcolemmal Ca2+ transport in streptozotocin-induced diabetic cardiomypathy in rat. Am J Physiol 253:E202-E207, 1987
Makino N, Panagia V, Gupta MP, Dhalla NS: Defects in sarcolemmal Ca2+ transport in hearts due to induction of calcium paradox. Circ Res 63:313–321, 1988
Nakanishi H, Makino N, Hata T, Matsui H, Yano K, Yanaga T: Sarcolemmal Ca2+ transport activities in cardiac hypertrophy caused by pressure overload. Am J Physiol 257:H349-H356, 1989
Mak IT, Misra HP, Weglicki WG: Temporal relationship of free radical induced lipid peroxidation and loss of latent enzyme activitity in highly enriched hepatic lysosomes. J Biol Chem 258:13733–13737, 1983
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275, 1951
Kurachi Y: The effects of intracellular protons on the electrical activity of single ventricular cells. Pflugers Arch 394:264–270, 1982
Meng HP, Pierce GN: Protective effects of 5-(N,N-dimethyl) amiloride on ischemia-reperfusion injury in hearts. Am J Physiol 258: H1615-H1619, 1990
Weiss RG, Lakatta EG, Gerstenblith G: Effects of amiloride on metabolism and contractility during reoxygenation in perfused rat hearts. Circ Res 66:1012–1022, 1990
Kleyman TR, Cragoe EJ Jr: Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105:1–21, 1988
Sandeaux R, Sandeaux J, Gavach C, Brun B: Transport of Na+ by monensin across bimolecular lipid membranes. Biochim Biophys Acta 684:127–132, 1982
Grinwald PM: Calcium uptake during post-ischemic reperfusion in the isolated rat heart: Influence of extracellular sodium. J Mol Cell Cardiol 14:359–365, 1982
Renlund DG, Gerstenblith G, Lakatta EG, Jacobus WE, Kallman CH, Weisfeldt ML: Perfusate sodium during ischemia modifies postischemic functional and metabolic recovery in the rabbit heart. J Mol Cell Cardiol 14:795–801, 1984
Bing OHL, Brooks WW, Messer JV: Heart muscle viability following hypoxia: Protective effect of acidosis. Science 180:1297–1298, 1973
Kitakaze M, Weisfeldt ML, Marban E: Acidosis during early reperfusion prevents myocardial stunning in perfused hearts. J Clin Invest 82:920–927, 1988
Neely JR, Grotyohann LW: Role of glycolytic products in damage to ischemic myocardium. Dissociation of adenosine triphosphate levels and recovery of function of reperfused ischemic hearts. Circ Res 55:816–824, 1984
Weglicki WB, Mak IT, Simic MG: Mechanisms of cardiovascular drugs as oxidants. J Mol Cell Cardiol 22:1199–1208, 1990
Bernier M, Hearse DJ, Manning AS: Reperfusion-induced arrhythmias and oxygen-derived free radicals: Studies with ‘antifree radical’ interventions and a free radical-generating system in the isolated perfused rat heart. Circ Res 58:331–340, 1986
Pallandi RT, Perry MA, Campbell TJ: Proarrhythmic effects of an oxygen-derived free radical generating system on action potentials recorded from guinea pig ventricular myocardium: A possible cause of reperfusion-induced arrhythmias. Circ Res 61:50–54, 1987
Janse MJ, Van Capelle FJL, Morsink H, Kleber AG, Wilms-Schopman F, Cardinal R, D'Alnoncourt CN, Durrer D: Flow of ‘injury’ current and patterns of excitation during early ventricular arrhythmias in acute regional myocardial ischemia in isolated porcine and canine hearts: Evidence for two different arrhythmogenic mechanisms. Circ Res 47:151–165, 1980
Boineau JP, Cox JL: Slow ventricular activation in acute myocardial infarction: A source of re-entrant premature ventricular contractions. Circulation 48:702–713, 1973
Imanishi S, Surawicz B: Automatic activity in depolarized guinea pig ventricular myocardium. Circ Res 39:751–759, 1976
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yano, Ki., Maruyama, T., Makino, N. et al. Effects of amiloride on the mechanical, electrical and biochemical aspects of ischemia-reperfusion injury. Mol Cell Biochem 121, 75–83 (1993). https://doi.org/10.1007/BF00928702
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00928702