Abstract
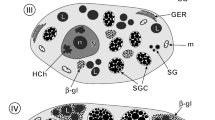
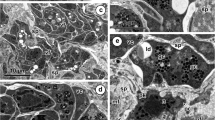
The effect of the microfilament inhibitor cytochalasin B (10 and 100 μg/ml) on the ultrastructure of adultFasciola hepatica was determined in vitro by scanning and transmission electron microscopy (SEM, TEM) using both intact flukes and tissue-slice material. SEM revealed that initial swelling of the tegument led to surface blebbing and limited areas of sloughing after 24 h treatment at 100 μg/ml. In the tegumental syncytium, basal accumulations of secretory bodies (especially T2s) were evident in the earlier time periods but declined with longer incubations, until few secretory bodies remained in the syncytium overall. Blebbing of the apical plasma membrane and occasional areas of breakdown and sloughing of the tegument were observed over longer periods of treatment at 100 μg/ml. In the tegumental cell bodies, the Golgi complexes gradually decreased in size and activity, and few secretory bodies were produced. In the later time periods, the cells assumed abnormal shapes, the cytoplasm shrinking in towards the nucleus. In the vitelline follicles, a random dispersion of shell protein globules was evident within the intermediate-type cells, rather than their being organized into distinct shell globule clusters. Disruption of this process was more severe at the higher concentration of 100 μg/ml and again was more evident in tissue-slice material. In the latter, after prolonged (12 h) exposure to cytochalasin B, the intermediate and mature vitelline cells were filled with loosely packed and expanded shell globule clusters, containing few shell protein globules. The mature vitelline cells continued to lay down “yolk” globules and glycogen deposits. Disruption of the network of processes from the nurse cells was evident at the higher concentration of cytochalasin. Spaces began to appear between the vitelline cells and grew larger with progressively longer incubation periods, and the cells themselves assumed abnormal shapes. A number of binucleate stem cells were observed in tissue-slice material at the longest incubation period (12 h).
Similar content being viewed by others
References
Abbas MK, Cain GD (1987) Actin and intermediate-sized filaments of the spines and cytoskeleton ofSchistosoma mansoni. Parasitol Res 73:66–74
Allison AC, Davies P, DePetris S (1971) Role of contractile microfilaments in macrophage movement and endocytosis. Nature New Biol 232:153–155
Atkinson C, Newsam RJ, Gull K (1980) Influence of the antimicrotubule agent, mebendazole, on the secretory activity of intestinal cells ofAscaridia galli. Protoplasma 105:69–76
Bennett CE, Hugnes DL, Harness E (1980)Fasciola hepatica: changes in tegument during killing of adult flukes surgically transferred to sensitized rats. Parasite Immunol 2:39–55
Bluemink JG (1978) Use of cytochalasins in the study of amphibian development. In: Tanenbaum SW (ed) Cytochalasins — biochemical and biological aspects. Elsevier/North-Holland, Amsterdam New York Oxford, pp 113–142
Borgers M, De Nollin S (1975) Ultrastructural changes inAscaris suum intestine after mebendazole treatment in vivo. J Parasitol 61:110–122
Borgers M, De Nollin S, De Brabander M, Thienpont D (1975a) Influence of the anthelmintic mebendazole on microtubules and intercellular organelle movement in nematode intestinal cells. Am J Vet Res 36:1153–1166
Borgers M, De Nollin S, Verheyen A, Vanparijs O, Thienpont D (1975b) Morphological changes in cysticerci ofTaenia taeniaeforms after mebendazole treatment. J Parasitol 61:830–843
Burgess TL, Kelly RB (1987) Constitutive and regulated secretion of proteins. Ann Rev Cell Biol 3:243–293
Butcher FR, Goldman RH (1974) Effect of cytochalasin B and colchicine on α-amylase release from rat parotid tissue slices. J Cell Biol 60:519–523
Carter SB (1972) The cytochalasins as research tools in cytology. Endeavour 31:77–82
Cohen C, Reinhardt B, Castellani L, Norton P, Stirewalt M (1982) Schistosome surface spines are “crystals” of actin. J Cell Biol 95:987–988
Cooper JA (1987) Effects of cytochalasin and phalloidin on actin. J Cell Biol 105:1473–1478
Davis AH, Blanton R, Klich P (1985) Stage and sex specific differences in actin gene expression inSchistosoma mansoni. Mol Biochem Parasitol 17:289–298
Fairweather I, Anderson HR, Threadgold LT (1986)Fasciola hepatica: tegumental changes induced in vitro by the deacetylated (amine) metabolite of diamphenethide. Exp Parasitol 62:336–348
Fairweather I, Anderson HR, Baldwin TMA (1987)Fasciola hepatica: tegumental surface alterations following treatment in vitro with the deacetylated (amine) metabolite of diamphenethide. Parasitol Res 73:99–106
Fairweather I, Anderson HR, Threadgold LT (1988)Fasciola hepatica: morphological changes in vitelline cells following treatment in vitro with the deacetylated (amine) metabolite of diamphenethide (DAMD). Int J Parasitol 18:1061–1069
Flanagan MD, Lin S (1980) Cytochalasins block actin filament elongation by binding to high affinity sites associated with Factin. J Biol Chem 255:835–838
Forte TM, Machen TE, Forte JG (1975) Ultrastructural and physiological changes in piglet oxyntic cells during histamine stimulation and metabolic inhibition. Gastroenterology 69:1208–1222
Hanna REB (1980a)Fasciola hepatica: an immunofluorescent study of antigenic changes in the tegument during development in the rat and the sheep. Exp Parasitol 50:155–170
Hanna REB (1980b)Fasciola hepatica: autoradiography of protein synthesis, transport, and secretion by the tegument. Exp Parasitol 50:297–304
Hanna REB, Threadgold LT (1975) Development of an in vitro technique for cytological investigations of slices ofFasciola hepatica: evaluation by morphological criteria. Int J Parasitol 5:321–331
Happich FA, Boray JC (1969) Quantitative diagnosis of chronic fascioliosis: 2. The estimation of dally total egg production ofFasciola hepatica and the number of adult flukes in sheep by faecal egg counts. Aust Vet J 45:329–331
Hockley DJ (1973) Ultrastructure of the tegument ofSchistosoma. Adv Parasitol 11:233–305
Irwin SWB, Threadgold LT (1970) Electron-microscope studies onFasciola hepatica. VIII. The development of the vitelline cells. Exp Parasitol 28:399–411
Isseroff H, Read CP (1969) Studies on membrane transport-VI. Absorption of amino acids by fascioliid trematodes. Comp Biochem Physiol 30:1153–1160
Isseroff H, Read CP (1974) Studies on membrane trasport-VIII. Absorption of monosaccharides byFacciola hepatica. Comp Biochem Physiol [A] 47:141–152
Kristen U, Lockhausen J (1983) Estination of Golgi membrane flow rates in ovary glands ofAptenia cordifolin using cytochalasin B. Eur J Cell Biol 29:262–267
Lacey E (1988) The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int J Parasitol 18:885–936
Lackie JM (1986) Cell movement and cell behaviour. Allen and Unwin, London
Lin S, Spudich JA (1974) Biochemical studies on the mode of action of cytochalasin B. J Biol Chem 249:5778–5783
Lin S, Lin DC, Flanagan MD (1978) Specificity of the effects of cytochalasin B on transport and motile processes. Proc Natl Acad Sci USA 75:329–333
MacGregor AN, Shore SJ (1990) Immunocytochemistry of cytoskeletal proteins in adultSchistosoma mansoni. Int J Parasitol 20:279–284
Matsumoto Y, Perry G, Levine RJC, Blanton R, Mahmoud AAF, Aikawa M (1988) Paramyosin and actin in schistosomal teguments. Nature 333:76–78
McGuire J, Moellmann G (1972) Cytochalasin B: effect on microfilaments and movement of melanin granules within melanocytes. Science 175:642–644
Mollenhauer HH, Morré DJ (1976) Cytochalasin B, but not colchicine, inhibits migration of secretory vesicles in root tips of maize. Protoplasma 87:39–48
Nève P, Ketelbant-Balasse P, Willems C, Dumont JE (1972) Effects of inhibitors of microtubules and microfilaments on dog thyroid slices in vitro. Exp Cell Res 74:227–244
Picton JM, Steer MW (1981) Determination of secretory vesicle production rates by dictyosomes in pollen tubes ofTradescantia using cytochalasin D. J Cell Sci 49:261–272
Picton JM, Steer MW (1983) Membrane recycling and the control of secretory activity in pollen tubes. J Cell Sci 63:303–310
Rao KH (1959) Observations on the Mehlis' gland complex in the liver flukeFasciola hepatica L. J Parasitol 45:347–351
Rappaport R (1986) Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol 105:245–281
Rogan MT, Threadgold LT (1984)Fasciola hepatica: tegumental alterations as a consequence of lectin binding. Exp Parasitol 57:248–260
Rosenfeld GC, McAllister E, Thompson WJ (1981) Cytochalasin inhibition of isolated rat gastric parietal cell function. J Cell Physiol 109:53–57
Salmon ED (1989) Cytokinesis in animal cells. Curr Opin Cell Biol 1:541–547
Schofield JG (1971) Cytochalasin B and release of growth hormone. Nature New Biol 234:215–216
Schroeder TE (1978) Cytochalasin B, cytokinesis, and the contractile ring. In: Tanenbaum SW (ed) Cytochalasins — biochemical and biological aspects. Elsevier/North-Holland, Amsterdam New York Oxford, pp 91–112
Shannon TM, Picton JM, Steer MW (1984) The inhibition of dictyosome vesicle formation in higher plant cells by cytochalasin D. Eur J Cell Biol 33:144–147
Skuce PJ, Fairweather I (1988)Fasciola hepatica: perturbation of secretory activity in the vitelline cells by the sodium ionophore monensin. Exp Parasitol 65:20–30
Skuce PJ, Fairweather I (1989)Fasciola hepatica: the effect of the sodium ionophore monensin on the adult tegument. Parasitol Res 75:223–232
Skuce PJ, Fairweather I (1990) The effect of the hydrogen ionophore closantel upon the pharmacology and ultrastructure of the adult liver flukeFasciola hepatica. Parasitol Res 76:241–250
Sinyth JD, Halton DW (1983) The Physiology of Trematodes. Cambridge University Press, Cambridge
Spooner BS (1978) Cytochalasins as probes inselected morphogenetic processes. In: Tanenbaum SW (ed) Cytochalasins — biochemical and biological aspects. Elsevier/North-Holland, Amsterdam New York Oxford, pp 65–89
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Stephenson W (1947) Physiological and histochemical observations on the adult liver fluke,Fasciola hepatica L.-III. Egg-shell formation. Parasitology 38:128–139
Stitt AW, Fairweather I, Johnston CF (1991a)Fasciola hepatica: disruption of spermatogenesis by the microfilament inhibitor cytochalasin B. Parasitol Res 77:123–128
Stitt AW, Fairweather I, Trudgett AG, Johnston CF, Anderson SML (1991b) Localisation of actin in the liver fluke,Fasciola hepatica. Parasitol Res (in press)
Tanenbaum SW (1978) Cytochalasins — biochemical and cell biological aspects. Elsevier/North-Holland, Amsterdam New York Oxford
Threadgold LT (1963) The tegument and associated structures ofFasciola hepatica. Q J Microsc Sci 104:505–512
Threadgold LT (1967) Electron microscope studies ofFasciola hepatica. III. Further observations on the tegument and associated structures. Parasitology 57:633–637
Threadgold LT (1982)Fasciola hepatica: stereological analysis of vitelline cell development. Exp Parasitol 54:352–365
Threadgold LT (1984) Parasitic platyhelminths. In: Bereiter-Hahn J, Matoltsy AG, Richards KS (eds) Biology of the integument, vol 1. Invertebrates. Springer, Berlin Heidelberg New York, pp 132–191
Threadgold LT (1985)Fasciola hepatica: interaction of the tegument with poly-l-lysine and enzymes. Exp Parasitol 59:222–230
Threadgold LT, Brennan GP (1978)Fasciola hepatica: basal infolds and associated vacuoles of the tegument. Exp Parasitol 46:300–316
Verheyen A, Borgers M, Vanparijs O, Thienpont D (1976) The effects of mebendazole on the ultrastructure of cestodes. In: Van den Bossche H (ed) Biochemistry of parasites and hostparasite relationships. Elsevier/North-Holland, Amsterdam, pp 605–618
Vail JD, Garrido J (1976) Actin-like filaments and membrane rearrangement in oxyntic cells. Proc Natl Acad Sci USA 73:4032–4036
Wessels NK, Spooner BS, Ash JF, Bradley MO, Luduena MA, Taylor EL, Wrenn JT, Yamada KM (1971) Microfilaments in cellular and developmental processes. Science 171:135–143
Williams JA, Wolff J (1971) Cytochalasin B in hibits thyroid secretion. Biochem Biophys Res Commun 44:422–425
Wilson RA, Barnes PE (1974) An in vitro investigation of dynamic processes occurring in the schistosome tegument using compounds known to disrupt secretory processes. Parasitology 68:259–270
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stitt, A.W., Fairweather, I. Fasciola hepatica: the effect of the microfilament inhibitor cytochalasin B on the ultrastructure of the adult fluke. Parasitol Res 77, 675–685 (1991). https://doi.org/10.1007/BF00928682
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00928682