Abstract
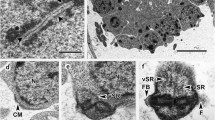
The fine structure of promastigotes ofLeptomonas samueli is described. This protozoan revealed several features in common with other trypanosomatids. A large membrane-bound cavity containing many vesicles was observed near the nucleus. Pinocytotic vesicles were seen arising from the membrane lining the flagellar pocket. They are associated with some microtubules which originate in the flagellar pocket region and extend toward the multivesicular structure. Morphometric analysis made on electron micrographs showed a mitochondrial relative volume of 0.11 and a peroxisome relative volume of 0.08. Determination of the number of sub-pellicular microtubules in different regions of the protozoon body show that the largest number is found in the region containing the Golgi complex. Carbohydrates were detected using the periodic-acid-thiosemicarbazide-silver proteinate technique. Reaction products were seen in the plasma membrane, in the membrane of the Golgi complex, and in the membranes which form the multivesicular structure. Two cytochemical methods were used to locate basic proteins. Using the ammoniacal silver method reaction products were seen only in the nucleus. With the ethanolic phosphotungstic acid method reaction products were seen in the microtubules which form the flagellum, in the peroxisome-like organelle, and at the region of adhesion of the flagellum to the cell body.
Similar content being viewed by others
References
Afzelius, B.A.: Ultrastructure of cilia and flagella. In: Handbook of molecular cytology, A. Lima de Faria, ed., pp. 1219–1242. Amsterdam-London: North-Holland Publishing Company 1969
Allen, R.D., David, G.B., Nomarski, G.: The Zeiss-Nomarski differential interference equipment for transmitted-light microscopy. Z. Wissen. Microsc. Techn.69, 193–221 (1969)
Anderson, W.A., Ellis, R.A.: Ultrastructure ofTrypanosoma lewisi. Flagellum, microtubules and the kinetoplast. J. Protozool.12, 483–499 (1965)
Bianchi, L., Rondaneli, E.G., Carasi, G., Gerna, G.: Endonuclear spindle in leptomonad ofLeishmania tropica. J. Parasitol.55, 1091–1092 (1969)
Brack, C.: Elektronenmikroskopische Untersuchungen zum Lebenszyklus vonTrypanosoma cruzi. Acta Trop. (Basel)25, 289–356 (1968)
Brooker, B.E.: The fine structure ofCrithidia fasciculata with special reference to the organelles involved in the ingestion and digestion of protein. Z. Zellforsch.116, 532–563 (1971)
Brooker, B.E.: The cell coat ofCrithidia fasciculata. Parasitology72, 259–267 (1976)
Bunn, M.M., Soares, T.C.B., Angluster, J., De Souza, W.: Effect of 2-deoxy-D-glucose onHerpetomonas samuelpessoai. Z. Parasitenkd.52, 245–256 (1977)
Carvalho, A.L.M.: Estudos sobre a posição sistemática e a transmissão de tripanosomatídeos encontrados emZelus leucogramus (Perty, 1834) (Hemiptera, Reduvidae). Rev. Patol. Trop.2, 223–274 (1973)
De Souza, W.: Cytochemical detection of carbohydrates in the Golgi complex ofLeptomonas pessoai. Z. Parasitenkd.48, 221–226 (1976)
De Souza, W., Brasil, R.P.: An electron microscopic and cytochemical detection of concanavalin A receptors on the cell membrane ofLeishmania braziliensis guyanensis. Z. Parasitenkd.50, 1–8 (1976)
De Souza, W., Meyer, H.: On the fine structure of the nucleus inTrypanosoma cruzi in tissue culture forms. Spindle fibers in the dividing nucleus. J. Protozool.21, 48–52 (1974)
De Souza, W., Meyer, H.: An electron microscopic and cytochemical study of the cell coat ofTrypanosoma cruzi in tissue cultures. Z. Parasitenkd.46, 179–187 (1975)
De Souza, W., Rossi, M.A., Kitajima, E.W., Santos, R., Roitman, I.: An electron microscopic study ofHerpetomonas sp. (Leptomonas pessoai). Can. J. Microbiol.22, 197–203 (1976)
Dwyer, D.M., Langreth, S.G., Dwyer, N.K.: Evidence for a polysaccharide surface coat in the developmental stages ofLeishmania donovani. A fine structure cytochemical study. Z. Parasitenkd.43, 227–249 (1974)
Frugulhetti, I.C.P., Rebelo, M.A.: Atividade de enzimas do ciclo do glioxalato emHerpetomonas samuelpessoai. Anais 4a. Reunião sobre Doença de Chagas, Caxambu, Brasil, p. 50, 1977
Gardner, P.J.: Pellicle-associated structures in the amastigote stage ofTrypanosoma cruzi andLeishmania species. Ann. Trop. Med. Parasitol.68, 167–176 (1974)
Gordon, M., Bensch, K.G.: Cytochemical differentiation of the guinea pig sperm flagellum with phosphotungstic acid. J. Ultrastruct. Res.24, 33–50 (1968)
Heywood, P., Weinman, D.: Mitosis in the hemoflagellateTrypanosoma cyclops. J. Protozool.25, 287–292 (1978)
Kusel, P.J., Moore, K.E., Weber, M.M.: The ultrastructure ofCrithidia fasciculata and morphological changes induced by growth in acriflavine. J. Protozool.14, 283–296 (1967)
MacRae, E.K., Meetz, G.D.: Electron microscopy of the ammoniacal silver reaction for histones in the erythropoietic cells of the chick. J. Cell Biol.45, 235–245 (1970)
Meyer, H., De Souza, W.: Electron microscopic study ofTrypanosoma cruzi periplast in tissue cultures. I. Number and arrangement of the peripheral microtubules in the various forms of the parasite's life cycle. J. Protozool.23, 385–390 (1976)
Milder, R., Deane, M.P.: Ultrastructure ofTrypanosoma conorhini in the crithidial phase. J. Protozool.14, 65–72 (1967)
Muse, K.E., Roberts, J.F.: Microbodies inCrithidia fasciculata. Protoplasma78, 343–348 (1973)
Souto-Padrón, T., De Souza, W.: Ultrastructural localization of basic proteins inTrypanosoma cruzi. J. Histochem. Cytochem.26, 349–358 (1978)
Steiger, R.: Some aspects of the surface coat formation inTrypanosoma brucei. Acta Trop. (Basel)28, 341–346 (1971)
Thiéry, J.P.: Mise en évidence des polysaccharides sur coupes fines en microscopie electronique. J. Microsc.6, 987–1018 (1967)
Vickerman, K., Preston, T.M.: Spindle microtubules in the dividing nuclei of trypanosomes. J. Cell Sci.6, 365–383 (1970)
Vickerman, K., Tetley, L.: Recent ultrastructural studies on trypanosomes. Ann. Soc. Belg. Med. Trop.57, 441–455 (1977)
Weibel, E.R.: Stereological principles for morphometry in electron microscopic cytology. Int. Rev. Cytol.26, 235–302 (1969)
Whight, K.A., Hales, H.: Cytochemistry of the pellicle of bloodstream forms ofTrypanosoma (Trypanozoon) brucei. J. Parasitol.56, 671–683 (1970)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Souto-Padrón, T., Gonçalves de Lima, V.M.Q., Roitman, I. et al. An electron microscopic and cytochemical study ofLeptomonas samueli . Z. Parasitenkd. 62, 127–143 (1980). https://doi.org/10.1007/BF00927859
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00927859