Abstract
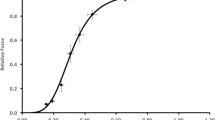
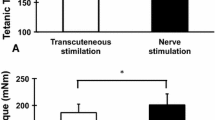
To examine the effect of short term intense activity on sarcoplasmic reticulum (SR) Ca2+ sequestering function, the gastrocnemius (G) muscles of 11 anaesthetized male rats (weight, 411±8 g,X±SE) were activated using supramaximal, intermittent stimulation (one train of 0.2 msec impulses per sec of 100 msec at 100 Hz). Homogenates were obtained from stimulated white (WG-S) and red (RG-S) tissues, assayed for Ca2+ uptake and maximal Ca2+ ATPase activity and compared to contralateral controls (WG-C, RG-C). Calcium uptake (nmoles/mg protein/min) determined using Indo-l and at [Ca2+]f concentrations between 300–400 nM was unaffected (p>0.05) by activity in both WG (6.14+0.43 vs 5.37+0.43) and RG (3.21+0.18 vs 3.07+0.20). Similarly, no effect (p>0.05) of contractile activity was found for maximal Ca2+ ATPase activity (μmole/mg protein/min) determined spectrophotometrically in RG (0.276+0.03 vs 0.278+0.02). In WG, Ca2+ ATPase activity was 15% higher in WG-S compared to WG-C (0.412+0.03 vs 0.385+0.04). Repetitive stimulation resulted in a reduction in tetanic tension of 74% (p<0.05) by 2 min in the G muscle. By the end of the stimulation period, ATP concentration was reduced (p<0.05) by 57% in the WG and by 47% in the RG. These results indicate that the repeated generation of maximal tetanic force, at least for short term periods, need not adversely affectin vitro homogenate determination of Ca2+ sequestering function in spite of severe alterations in energy potential and that some other mechanism must be involved to explain the depression in Ca2+ uptake and Ca2+ ATPase activity previously noted with short term intense exercise.
Similar content being viewed by others
References
Byrd SK: Alterations in the sarcoplasmic reticulum: a possible link to exercise-induced muscle damage. Med Sci Sports Ex 24: 531–536, 1992
Westerblad H, Lee JA, Lannergren J, Allen DG: Cellular mechanisms of fatigue in skeletal muscle. Am J Physiol 261: C195–C209, 1991
Byrd SK, McCutcheon LJ, Hodgson DR, Gollnick PD: Altered sarcoplasmic reticulum function after high intensity exercise. J Appl Physiol 67: 2072–2077, 1989
Byrd SK, Bode AK, Klug GA: Effects of exercise of varying duration on sarcoplasmic reticulum function. J Appl Physiol 66: 1383–1388, 1989
Favero TG, Pessah IN, Klug GA: Prolonged exercise reduces Ca2+-release in rat skeletal muscle sarcoplasmic reticulum. Pflügers Arch 422: 472–475, 1993
Belcastro AN, Rossiter M, Low MP, Sopper MM: Calcium activation of sarcoplasmic reticulum ATPase following strenuous activity. Can J Physiol Pharmacol 59: 1214–1218, 1981
Fitts RH, Courtright JB, Kim DH, Witzmann FA: Muscle fatigue with prolonged exercise: contractile and biochemical alterations. Am J Physiol 242: C65–C73, 1982
Heilman C, Pette D: Molecular transformation in sarcoplasmic reticulum of fast twitch muscle by electro-stimulation. Europ J Biochem 93: 437–446, 1979
Leberer E, Hartner KT, Pette D: Reversible inhibition of sarcoplasmic reticulum Ca-ATPase by altered neuromuscular activity in rabbit fast-twitch muscle. Europ J Biochem 162: 555–561, 1987
Rapundalo ST, Briggs FN, Feher JJ: Effects of ischemia on the isolation and function of canine cardiac sarcoplasmic reticulum. J Mol Cell Cardiol 18: 837–851, 1986
Simonides WS, van Hardeveld C: An assay for sarcoplasmic reticulum Ca2+-ATPase activity in muscle homogenates. Anal Biochem 191: 321–331, 1990
Korge P, Byrd SK, Schenk JO: Use of the Ca2+ electrode for measuring the rate of Ca2+ uptake in muscle homogenates. A practical approach. J Appl Physiol Submitted: 1994
O'Brien PJ, Shen H, Weiler J, Mirsalami M, Julian R: Myocardial Ca-sequestration failure and compensatory increase in Ca-ATPase with congestive cardiomyopathy: kinetic characterization by a homogenate microassay using real-time ratiometric indo-I spectrofluorometry. Mol Cell Biochem 102: 1–12, 1991
Westerblad H, Lannergren J: Slowing of relaxation during fatigue in single mouse muscle fibres. J Physiol 434: 323–336, 1991
Cady EB, Elshoue H, Jones DA, Moll A: The metabolic causes of slow relaxation in fatigued human skeletal muscle. J Physiol 418: 327–337, 1989
Thompson LV, Balog EM, Riley DA, Fitts RH: Muscle fatigue in frog semitendinosus: alterations in contractile function. Am J Physiol 262: C1500–C1506, 1992
Meyer RA, Terjung RA: Difference in ammonia and adenylate metabolism in contracting slow and fast muscle. Am J Physiol 237: C111–C118, 1979
Dudley GA, Terjung RL: Influence of acidosis on AMP de-aminase activity in contracting fast-twitch muscle. Am J Physiol 248: C43–C50, 1985
MacIntosh BR, Gardiner PF: Post tetanic potentiation and skeletal muscle fatigue: Interactions with caffeine. Can J Physiol Pharmacol 65: 260–268, 1987
de Haan A, Jones DA, Sargeant AJ: Changes in velocity of shortening power output and relaxation rate during fatigue of medial gastrocnemius muscle. Pflugers Arch 415: 433–439, 1990
Harris RC, Hultman E, Nordesjö L-O: Glycolytic intermediates and high energy phosphates determined in biopsy samples of musculus femoris of man at rest. Scand J clin Lab Invest 33: 102–120, 1974
Lowry OH, Passonneau JV: A flexible system of enzymatic analysis New York, Academic Press, pp 146–218, 1972
Green HJ, Hughson RL, Thomson JA, Sharratt MT: Supramaximal exercise after training-induced hypervolemia. I. Gas exchange and acid-base balance. J Appl Physiol 62: 1944–1953, 1987
Ingebretson DC, Bakken AM, Segadal L, Farstad M: Determination of adenine nucleotides and ionsine in human myocardium by ion pair reversed phase high performance liquid chromatography. J Chromatogr 242: 119–126, 1982
Green HJ, Sutton J, Young P, Cymerman A, Houston CS: Operation Everest II: muscle energetics during maximal exhaustive exercise. J Appl Physiol 66(1): 142–150, 1989
Dudley GA, Tullson PC, Terjung RL: Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem 262: 9109–9114, 1987
Sahlin K, Harris R, Hultman E: Creatine kinase equilibrium and lactate content compared with muscle pH in tissue samples obtained after isometric exercise. Biochem J 152: 173–180, 1975
Grynkiewicz G, Poenie M, Tsien RY: A new generation of Ca2+-indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985
O'Brien PJ: Calcium sequestration by isolated sarcoplasmic reticulum: real time monitoring using ratiometric dual emission spectrofluorometry and the fluorescent calcium binding indo-I. Mol Cell Biochem 94: 113–119, 1990
Fabiato A: Ca2+ buffering: computer programs and simulations, in J.G. McCormack, P.H. Cobbold (eds). Cellular Calcium. A Practical Approach. New York, Oxford University Press, 1991, pp 159–176
Le Rumeur E, Le Moyel L, Chagneau F, Levasseur M, Toulouse P, Le Bars R, De Certaines J: Phosphocreatine and pH recovery without restoration of mechanical function during prolonged activity of rat gastrocnemius muscle: anin vivo 31p NMR study. Arch Internat de Physiol et de Biochim 97: 381–388, 1989
Meyer RA, Terjung RL: AMP deamination and IMP reamination in working skeletal muscle. Am J Physiol 239: C32–C38, 1980
Hood DA, Parent G: Metabolic and contractile responses of fast-twitch muscle to 10 Hz stimulation. Am J Physiol 260: C832–C840, 1991
Kim DH, Witzman FA, Fitts RH: A comparison of the sarcoplasmic reticulum function in fast and slow skeletal muscle using crude homogenates and isolated vesicles. Life Sci 28: 2223–2229, 1981
Tate C, Hamra M, Shin G, Taffet G, McBride P, Entman M: Canine cardiac sarcoplasmic reticulum is not altered with endurance exercising training. Med Sci Sports Ex 25: 1246–1257, 1993
Metzger JM, Moss RL: pH modulation of the kinetics of a Ca2+-sensitive cross-bridge state transition in mammalian single skeletal muscle fibres. J Physiol (London) 428: 751–764, 1990
Blinks JR, Rudel R, Taylor SR: Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol (London) 277: 291–293, 1978
Green HJ, Düsterhöft S, Dux L, Pette D: Metabolite patterns related to exhaustion, recovery and transformation of chronically stimulated rabbit fast-twitch muscle. Pflügers Arch 420: 3S9–366, 1992
Mandell F, Kranias EG, DeGende AC, Sumida M, Schwartz A: The effect of pH on the transient-state kinetics of Ca2+−Mg2+ ATPase of cardiac sarcoplasmic reticulum. A comparison with skeletal sarcoplasmic reticulum. Circ Res 50: 310–317, 1982
Inesi G, Millman M, Eletr S: Temperature induced transitions of function and structure in sarcoplasmic reticulum membranes. J Mol Biol 81: 483–504, 1973
Dossett-Mercer J, Green HJ, Chin E, Grange F: Preservation of sarcoplasmic reticulum Ca2+ sequestering function in homogenates of different fibre type composition following sprint activity. Can J Physiol Pharmacol 76: 2586–2593, 1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dossett-Mercer, J., Green, H., Chin, E.R. et al. Failure of short term stimulation to reduce sarcoplasmic reticulum Ca2+-ATPase function in homogenates of rat gastrocnemius. Mol Cell Biochem 146, 23–33 (1995). https://doi.org/10.1007/BF00926877
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00926877