Abstract
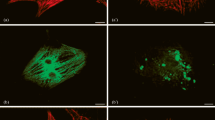
Immunocytochemical investigation was performed on the cytoskeletal proteins in cardiac tissue of the cardiomyopathic hamster. Male cardiomyopathic UM-X7.1 hamsters at 180 days of age (n=8) and age- and sex-matched normal BIO-RB hamsters (n=8) were used in this study. Immunofluorescence microscopy using monoclonal antibodies against desmin, α-actinin, titin, and vincullin was employed. The heart weight to body weight ratio was significantly increased in the heart of cardiomyopathic hamster compared with that of normal hamster. In cardiomyopathic hamster, the left ventricular cavity was markedly dilated. Light microscopically, hypertrophy and atrophy of myocytes and myocardial fibrosis were prominently observed in cardiomyopathic myocardium. Immunocytochemically, desmin, α-actinin and titin showed the cross striations along the myofibers in normal myocardium. In contrast, in cardiomyopathic myocardium, desmin was irregularly distributed in myocytes and the amount of desmin was increased. Loss of cross striations of α-actinin and titin were frequently observed. Immunofluorescence against vinculin was not significantly altered. We conclude that the alterations of cytoskeletal proteins in myocardial cells may relate to decreased myocardial function in cardiomyopathic hamster failing heart.
Similar content being viewed by others
References
Jasmin G, Eu HY: Cardiomyopathy of hamster dystrophy. Ann NY Acad Sci 317: 46–58, 1979
Jasmin G, Proschek L: Hereditary polymyopathy and cardiomyopathy in the Syrian hamster. I. Progression of heart and skeletal muscle lesions in the UM-X7.1 line. Muscle and Nerve 5: 20–25, 1982
Bajusz E: Hereditary cardiomyopathy: A new disease model. Am Heart J 77: 686–696, 1969
Bajusz E, Baker JR, Nixon CW, Homburger F: Spontaneous hereditary myocardial degeneration and congestion heart failure in a strain of Syrian hamster. Ann NY Acad Sci 156: 105–129, 1969
Schaper J, Froede R, Hein St, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N: Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation 83: 504–514, 1991
Baandruo U, Olsen EGJ: Critical analysis of endomyocardial biopsies from patients suspected of having cardiomyopathy: I. Morphological and morphometric aspects. Br Heart J 45: 475–486, 1981
Weibel ER: Stereology principles for morphometry in electron microscopic cytology. Int Rev Cytol 26: 235–302, 1969
Büchner F, Onishi S, Wada A: Cardiomyopathy associated with systemic myopathy. Genetic defect of actomyosin influencing muscular structure and function. Urban and Schwarzengerg, Baltimore Munich, 1978
Kato M, Nagano M: Experimental animal models of cardiomyopathy. In: L. Opie, T. Sugimoto (eds). Cardiomyopathy update 4: Metabolic and molecular aspects of cardiomyopathy. University of Tokyo Press, Tokyo, pp 69–81, 1991
Kawaguchi N, Wada A, Onishi S: Morphometric analysis of the heart in cardiomyopathic hamster (abstract). J Mol Cell Cardio 23 (suppl II): S35, 1991
Paterson RA, Layberry RA, Nadkarni, BB: Cardiac failure in the hamster. A biochemical and electron microscopic study. Lab Invest 26: 755–799, 1972
Strobeck JE, Factor SM, Bhan A, Sole M, Liew CC, Fein F, Sonnenblick EH: Hereditary and acquired cardiomyopathies in experimental animals: Mechanical, biochemical, and structural features. Ann NY Acad Sci 317: 59–88, 1979
Lazarides E, Granger BL, Gard DL, O'Connor CM, Breckler J, Price M, Danto SI: Desmin- and vimentin-containing filaments and their role in the assembly of the Z disk in muscle cells. Cold Spring Harbour Symp Quant 46: 351–378, 1982
Carlsson E, Kjorell U, Thornell LE: Differentiation of the myofibrils and the intermediate filament system during postnatal development of the rat heart. Eur J Cell Biol 27: 62–73, 1982
Thornell LE, Johansson B, Eriksson A, Lehto VP, Virtamen I: Intermediate filament and associated proteins in the human heart: An immunofluorescence study of normal and pathological hearts. Er Heart J 5 (suppl F): 231–241, 1984
Porte A, Stoeckel ME, Sacrez A, Batzenschlager A: Unusual familial cardiomyopathy with storage of intermediate filaments in the cardiac muscular cells. Virchows Arch A Path Anat and Histol 386: 43–58, 1980
Stoeckel ME, Osborn M, Porte A, Sacrez A, Batzenschlager A, Weber K: An unusual familial cardiomyopathy characterized by aberrant accumulations of desmin-type intermediate filaments. Virchows Arch A Path Anat and Histol 393: 53–60, 1981
D'Amati G, Kahn HJ, Butany J, Silver MD: Altered distribution of desmin filaments in hypertrophic cardiomyopathy: An immunohistochemical study. Modern Pathology 5: 165–168, 1992
Tokuyasu KT, Dutton AH, Singer SJ: Immunoelectron microscopic studies of desmin (skeletin) localization and intermediate filament organization in chicken cardiac muscle. J Cell Biol 96: 1736–1742, 1983
Furst DO, Osborn M, Nave R, Weber K: The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: A map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol 106: 1563–1572, 1988
Tokuyasu KT, Dutton AH, Geiger B, Singer SJ: Ultrastructure of chicken cardiac muscle as studies by double immunolabelling in electron microscopy. Proc Natl Acad Sci USA 78: 7619–7623, 1981
Pardo JV, Siliciano JD, Craig SW: Vinculin is a component of an extensive network of myofibril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol 97: 1081–1088, 1983
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawaguchi, N., Fujitani, N., Schaper, J. et al. Pathological changes of myocardial cytoskeleton in cardiomyopathic hamster. Mol Cell Biochem 144, 75–79 (1995). https://doi.org/10.1007/BF00926743
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00926743