Abstract
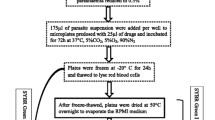
Isolates ofPlasmodium falciparum from villagers in central Sudan were tested for chloroquine and mefloquine sensitivity using the WHO microtechnique procedure and a modified 48-h in vitro test for drug resistance. No drug-resistant strains were noted. In the WHO procedure, in which parasites were cultivated in the presence of the patient's plasma, 72% of the isolates failed to mature to the schizont stage, but when infected erythrocytes were washed free of the patient's plasma and cultivated in pooled nonimmune serum only 28.8% of the isolates failed to develop to the schizont stage. In subsequent experiments, sera fromP. falciparum-infected patients or from noninfected “immune” adults were used to supplement standard in vitro test plates which contained parasites of known chloroquine sensitivities. Sera from malaria-infected patients or from immune adults retarded parasite development in the presence or absence of drug. The effect of these humoral factors and the antimalarial drugs was additive. The replacement of the patient's plasma with nonimmune serum in drug sensitivity tests performed with African isolates is recommended.
Similar content being viewed by others
References
Aronsson B, Bengtsson E, Björkman A, Pehrson PO, Rombo L, Wahlgren M (1981) Chloroquine-resistant falciparum malaria in Madagascar and Kenya. Ann Trop Med Parasitol 75:367–373
Campbell CC, Chin W, Collins WE, Tentsch SM, Moss DM (1979) Chloroquine-resistantPlasmodium falciparum from East Africa. Cultivation and sensitivity of the tanzania I/CDC strain from an American tourist. Lancet II:1151–1154
Capps TC, Jensen JB (1983) Storage requirements for erythrocytes used to culturePlasmodium falciparum. J Parasitol 69:158–162
Center for Disease Control (1978) Chloroquine-resistant malaria acquired in Kenya and Tanzania-Denmark, Georgia, New York. CDC Morbidity and Mortality Weekly Rep 27:463–464
Desjardins RD, Canfield CJ, Haynes JD, Chulay JD (1979) Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother 16:710–718
Divo AA, Jensen JB (1982) Studies on serum requirements for the cultivation ofPlasmodium falciparum. 1. Animal sera. Bull WHO 60:565–569
Geary TG, Jensen JB (1983) Lack of cross-resistance to 4-aminoquinolines in chloroquine-resistantPlasmodium falciparum in vitro. J Parasitol 69:97–105
Jensen JB, Trager W (1977)Plasmodium falciparum in culture. Use of outdated erythrocytes and description of the candle jar method. J Parasitol 63:883–886
Jensen JB, Trager W (1978)Plasmodium falciparum in culture: establishment of additional strains. Am J Trop Med Hyg 27:743–746
Jensen JB, Capps TC, Carlin JM (1981) Clinical drug-resistant falciparum malaria acquired from cultured parasites. Am J Trop Med Hyg 30:523–525
Jensen JB, Boland MT, Akood MA (1982a) Induction of crisis forms in culturedPlasmodium falciparum with human immune serum from Sudan. Science 216: 743–746
Jensen JB, Boland MT, Hayes M, Akood MA (1982b)Plasmodium falciparum: A rapid assay for in vitro inhibition due to human serum from residents of malarious areas. Exp Parasitol 54:416–424
Jensen JB, Boland MT, Allan JS, Carlin JM, Vande Waa JA, Divo AA, Akood MA (1983) Association between human serum-induced crisis forms in culturedPlasmodium falciparum and clinical immunity to malaria in Sudan. Infect Immun 33:83–89
Jensen JB, Hoffman SL, Boland MT, Akood MA, Laughlin LW Kurniawan L, Marwoto HA (1984) Comparison of immunity to malaria in Sudan and Indonesia: Crisis-form versus merozoite-invasion inhibition. Proc Natl Acad Sci USA 81:922–925
Kean BH (1979) Chloroquine-resistant falciparum malaria from Africa. JAMA 241:395–396
Kouznetsov RL, Rooney W, Wernsdorfer WH, El Gaddal AA, Payne D, Abdalla RE (1980) Use of the in vitro microtechnique for the assessment of drug sensitivity ofPlasmodium falciparum in Sennar, Sudan. Bull WHO 58:785–789
Lambros C, Vanderberg JP (1979) Synchronization ofPlasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420
Nguyen-Dinh P, Trager W (1980)Plasmodium falciparum in vitro: Determination of chloroquine sensitivity of three new strains by a modified 48-hour test. Am J Trop Med Hyg 29:339–342
Omer AHS (1978) Response ofPlasmodium falciparum in Sudan to oral chloroquine. Am J Trop Med Hyg 27:853–857
Onori E, Payne D, Grab B, Horst HI, Almeida Franco J, Joia H (1982) Incipient resistance ofPlasmodium falciparum to chloroquine among a semi-immune population of the United Republic of Tanzania. I. Results of in vivo and in vitro studies and of an ophathalmological survey. Bul WHO 60:77–87
Schwartz IK, Campbell CC, Payne D, Khatib OJ (1983) In vivo and in vitro assessment of chloroquine-resistantPlasmodium falciparum malaria in Zanzibar. Lancet I:1003–1005
Spencer HC, Masaba SC, Kiaraho D (1982) Sensitivity ofPlasmodium falciparum isolates to chloroquine in Kisumu and Malindi, Kenya. Am J Trop Med Hyg 31:902–906
Taliaferro WH, Taliaferro LG (1944) The effect of immunity on the asexual reproduction ofPlasmodium brasilianum. J Infect Dis 75:1–32
Wernsdorfer WH (1980) Field evaluation of drug resistance in malaria. In vitro micro-test. Acta Trop 37:222–227
World Health Organization (1973) Chemotherapy of malaria and resistance to antimalarials. WHO Tech Rep Ser No 529
Yisunsri L, Rieckmann K (1980) In vitro microtechnique for determining the drug susceptibility of cultured parasites ofPlasmodium falciparum. Trans R Soc Trop Med Hyg 74809–810
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carlin, J.M., Vande Waa, J.A., Jensen, J.B. et al. African serum interference in the determination of chloroquine sensitivity inPlasmodium falciparum . Z. Parasitenkd. 70, 589–597 (1984). https://doi.org/10.1007/BF00926589
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00926589