Abstract
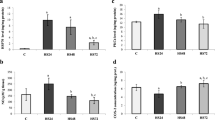
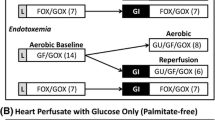
It was recently reported that in rats exposure to heat shock leads to appearance of a myocardial heat shock protein (HSP 70) and to an increase in myocardial catalase activity. This correlated with an improvement in post-ischemic function either in Langendorff-perfused hearts after low-flow ischemia or in working hearts after short-term, no-flow ischemia. We investigated the effect of the same hyperthermic treatment on functional recovery from no-flow ischemia of various durations in isolated working rat hearts performing at high or low external workloads. Rats were heated to core temperature of 42° C for 15 min. No significant protein oxidation (% oxidized methionine) was observed 2.5 hr after treatment. A protein with migration characteristics similar to HSP 70 was observed in hearts of heat shocked rats 24 hr after this treatment while their myocardial catalase activity was not increased. Hearts of similarly treated rats were excised 24 hr after hyperthermia and perfused in a working mode with Krebs-Henseleit buffer (1.25 mM Ca2+, 11 mM glucose). At 15 cm H2O preload and 100 cm H2O afterload after 30 min no-flow ischemia, control hearts recovered to 36.9%, 2%, 47.6%, and 21.5% of the preischemic values of heart rate-peak systolic pressure product (RPP), aortic output, coronary flow, and cardiac output, respectively. After only 25 min of ischemia the respective recovered values were 61.6%, 11.5%, 58.7%, and 33.5%. Throughout the recovery period these hemodynamic values were consistently higher in hearts of heat shocked animals than in those of control hearts but the differences were not statistically significant. After 25 min ischemia only 2 out of 7 control hearts recovered some aortic output, whereas in the heat shocked animals all 5 hearts recovered. After only 20 min of no-flow ischemia and at a lower workload (12.5 cm H2O preload and 75 cm H2O afterload), control hearts recovered to 85.1% of RPP, 54.1% of aortic output, and 68.3% of cardiac output. None of these variables was significantly improved by heat shock pretreatment. In summary, we were unable to demonstrate a similar degree of protective effect of heat shock pretreatment as compared to other reports where both HSP 70 and increased catalase activity were present. The reason(s) could be related to lack of induction of myocardial catalase activity in our study.
Similar content being viewed by others
References
Aebi H: Catalase. Methods of Enzymology 105: 121–126, 1984
Baker JC, Jacobson MK: Alteration of adenyl dinucleotide metabolism by environmental stress. Proc Natl Acad Sci USA 83: 2350–2352, 1986
Baker JE, Felix CC, Olinger GN, Kalyanaraman B: Myocardial ischemia and reperfusion: Direct evidence for free radical generation by electron spin resonance spectroscopy. Proc Natl Acad Sci USA 85: 2786–2789, 1988
Bilinski T, Krawiec Z, Litwinska J, Blaszczynski M: Mechanisms of oxygen toxicity as revealed by studies of yeast mutants with changed response to oxidative stress. In: PA Cerutti, I Fridovich, JM McCord (eds) Oxy-Radicals in Molecular Biology and Pathology. Alan R. Liss Inc., New York, 1988, pp 109–123
Bolli R, Jeroudi MO, Patel BS, Dubose BS, Lai EK, Roberts R, McCay PB: Direct evidence that oxygen-derived free radicals contribute to post-ischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci USA 86: 4695–4699, 1989
Bouchner BR, Lee PC, Wilson SW, Cutler CW, Ames BN: AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell 37: 225–232, 1984
Brown JM, Grosso MA, Terada LS, Whitman GJR, Banerjee A, White CW, Harken AH, Repine JE: Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci USA 86: 2525–2529, 1989
Chary P, Natvig DO: Evidence for three differentially regulated catalase genes in Neurospora crassa: Effects of oxidative stress, heat shock, and development. J Bacteriol 171: 2646–2652 1989
Currie RW, Karmazyn K, Kloc M, Mailer K: Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res 63: 543–549, 1988
Currie RW, Karmazyn M: Improved post-ischemic ventricular recovery in the absence of changes in energy metabolism in working rat hearts following heat-shock. J Mol Cell Cardiol 22: 631–636, 1990
Currie RW, Ross BM, Davis TA: Induction of the heat shock response in rats modulates heart rate, creatine kinase and protein synthesis after a subsequent hyperthermic treatment. Cardiovascular Res 24: 87–93, 1990
Currie RW, Karmazyn M, Tanguay RM: Induction of the heat shock response in rat hearts and acquisition of enhanced post ischemic ventricular recovery. In: B Maresca, S Lindquist (eds) Heat Shock. Proceedings of an International Workshop Ravello, Italy. IIGB Press, 1990, pp 104–105
Devous MD, Lewandowski ED: Inosine preserves ATP during ischemia and enhances recovery during reperfusion. Am J Physiol 253: H1224-H1233, 1987
Dillmann WH, Mehta HB, Barrieux A, Guth BD, Neeley WE, Ross J: Ischemia of the dog heart induces the appearance of a cardiac mRNA coding for a protein with migration characteristics similar to heat-shock/stress protein 71. Circ Res 59: 110–114, 1986
Duan J, Karmazyn M: Relationship between oxidative phosphorylation and adenine nucleotide translocase activity of two populations of cardiac mitochondria and mechanical recovery of ischemic hearts following reperfusion. Can J Physiol Pharmacol 67: 704–709, 1989
Eley D, Korecky B, Fliss H: Dithiothreitol restores contractile function to oxidant-injured cardiac muscle. Am J Physiol 257: H1321-H1325, 1989
Fliss H, Weissbach H, Brot N: Oxidation of methionine in proteins of activated human neutrophils. Proc Natl Acad Sci USA 80: 7160–7164, 1983
Hass MA, Massaro D: Regulation of the synthesis of superoxide dismutases in rat lungs during oxidant and hyperthermic stresses. J Biol Chem 263: 776–781, 1988
Hightower L, White FP: Cellular responses to stress: Comparison of a family of 71–73 kilodalton proteins rapidly synthesized in rat tissue slices and canavanine-treated cells in culture. J Cell Physiol 108: 261–275, 1981
Josephson RA, Silverman HS, Lakatta EG, Stern MD, Zweier JL: Study of the mechanisms of hydrogen peroxide and hydroxyl free radical-induced cellular injury and calcium overload in cardiac myocytes. J Biol Chem 266: 2354–2361, 1991
Kagawa Y, Ohta S: Regulation of mitochondrial ATP synthesis in mammalian cells by transcriptional control. Int J Biochem 22: 219–229, 1990
Karmazyn M, Mailer K, Currie RW: Acquisition and decay of heat-shock-enhanced postischemic ventricular recovery. Am J Physiol 259: H424-H431, 1990
Kaminishi T, Matsuoka T, Yanagishita T, Kako KJ: Increase vs decrease of calcium uptake by isolated heart cells induced by H2O2 vs HOCl. Am J Physiol 256: C598-C607, 1989
Lopaschuk GD, Wall SR, Olley PM, Davies N: Etomoxir, a cannitine palmitoyltransferase inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circ Res 63: 1036–1043, 1988
Lopaschuk GD, Spafford M, Davies NJ, Wall SR: Glucose and palmitate oxidation in isolated working rat hearts reperfused following a period of transient global ischemia. Circ Res 66: 546–553, 1989
McVeigh JJ, Lopaschuk GD: Dichloroacetate stimulation of glucose oxidation improves recovery of ischemic rat hearts. Am J Physiol 28: H1079–1085, 1990
Morgan RW, Christman MF, Jacobson FS, Storz G, Ames B: Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci USA 83: 8059–8063, 1986
Myers CL, Weiss SJ, Kirsh MM, Shlafer M: Involvement of hydrogen peroxide and hydroxyl radical in the ‘oxygen paradox’: reduction of creatine kinase release by catalase allopurinol or deferoxanine, but not by superoxide dismutase. J Mol Cell Cardiol 17: 675–684, 1985
Neely JR, Grotyohan LW: Role of glycolytic products in damage to ischemic myocardium. Circ Res 55: 816–824, 1984
Nemali MR, Usuda N, Reddy K, Oyasu K, Hashimoto T, Osumi T, Rao MS, Reddy JL: Comparison of constitutive and inducible levels of expression of peroxisomal beta-oxidation and catalase genes in liver and extrahepatic tissues of rat. Cancer Res 48: 5316–5324, 1988
Nohl H, Mordon W: The metabolic fate of mitochondrial hydrogen peroxide. Int J Biochem 111: 203–210, (1980)
O'Farrell P: High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250: 4007–4021, 1975
Panagiotopoulos S, Daly MJ, Nayler WG: Effect of acidosis and alkalosis on post-ischemic Ca gain in isolated rat heart. Am J Physiol 258: H821-H828, 1990
Paulson DJ, Noonan JJ, Ward KM, Sherratt SHA, Shug AL: Effects of POCA on metabolism and function in the ischemic rat heart. Basic Res Cardiol 81: 180–187, 1986
Paulson D, Kopp SJ, Peace DG, Tow JP: Improved postischemis recovery of cardiac pump function in exercised trained diabetic rats. J Appl Physiol 65: 187–193, 1988
Privalle CT, Fridovich I: Induction of superoxide dismutase in Escherichia coli by heat shock. Proc Natl Acad Sci USA 84: 2723–2726, 1987
Semsei I, Rao G, Richardson A: Changes in the expression of superoxide dismutase and catalase as a function of age and dietary restriction. Biochem Biophys Res Commun 164: 620–625, 1989
Storz G, Tartaglia LA, Ames BN: Transcriptional regulator of oxidative stress-inducible genes: Direct activation by oxidation. Science 248: 189–194, 1990
Tani M: Mechanisms of Ca2+ overload in reperfused ischemic myocardium. Annu Rev Physiol 52: 543–559, 1990
Tani M, Neely, JR: Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+−Na+ and Na+−Ca2+ exchange. Circ Res 65: 1045–1056, 1989
Tani M, Neely JR: Mechanisms of reduced reperfusion injury by low Ca2+ and/or high K+. Am J Physiol 258:H1025–1031, 1990
Thayer WS: Role of catalase in metabolism of hydrogen peroxide by the perfused rat heart. FEBS Lett 202:137–140, 1986
Wall SR, Lopaschuk GD: Glucose oxidation rates in fatty acidperfused isolated working hearts from diabetic rats. Biochim Biophys Acta 1006:97–103, 1989
Weiss RG, Lakatta EG, Gerstenblith G: Effects of amiloride on metabolism and contractility during reoxygenation in perfused rat hearts. Circ Res 66:1012–1022, 1990
Werns SW, Lucchesi BR: Leukocytes, oxygen radicals, and myocardial injury due to ischemia and reperfusion. J Free Rad Biol Med 4:31–37, 1988
Zweier JL, Kuppsamy P, Lutty GA: Measurement of endothelial cell free radical generation: Evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci USA 85:4046–4050, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wall, S.R., Fliss, H. & Korecky, B. Role of catalase in myocardial protection against ischemia in heat shocked rats. Mol Cell Biochem 129, 187–194 (1993). https://doi.org/10.1007/BF00926367
Issue Date:
DOI: https://doi.org/10.1007/BF00926367