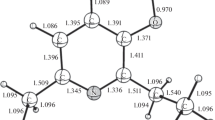
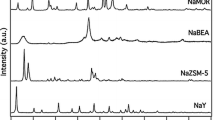
Conclusions
-
1.
The total concentrations of hydrogen (in the form of H2O, OH, and H3O+) in CaX and CaY zeolites, evacuated at 10−4 torr and 300, 400, or 500°, were determined by the method of isotopic exchange with D2.
-
2.
There is a threshold degree of exchange of Na+ for Ca2+, after which a rapid increase in the content of protium and the catalytic activity of zeolites in reactions of the carbonium ion type is observed.
-
3.
The mobility of H, as well as its thermal stability, increase with increasing Ca2+ content and mole ratio SiO2/Al2O3 in zeolites of the faujasite type.
-
4.
For calcium zeolites prepared from NaY, produced by hydrothermal synthesis and dealumination, the content and mobility of protium are close, which indicates identity of samples prepared by different methods.
Similar content being viewed by others
Literature cited
J. T. Richardson, J. Catalysis,9, 182 (1967).
P. B. Venuto and P. S. Landis, Advances in Catal. and Related Subjects,18, 259 (1968).
Kh. M. Minachev, Kinetika i Kataliz,11, 414 (1970).
V. G. Gvakhariya, V. I. Kvlividze, V. F. Kiselev, M. B. Pylova, and G. V. Tsitsishvili, Dokl. Akad. Nauk SSSR,188, 379 (1969).
V. G. Gvakhariya, V. I. Kvlividze, A. A. Kubasov, B. V. Romanovskii, K. V. Topchieva, and G. V. Tsitsishvili, Kinetika i Kataliz,10, 1392 (1969).
Kh. M. Minachev, R. V. Dmitriev, Ya. I. Isakov, and O. D. Bronnikov, Kinetika i Kataliz,12, 712 (1971).
T. Imanaka, Y. Okamoto, K. Takahata, and S. Teranishi, Bull. Chem. Soc. Japan,45, 366 (1972).
Kh. M. Minachev, R. V. Dmitriev, O. D. Bronnikov, V. I. Garanin, and T. A. Novruzov, Kinetika i Kataliz,13, 1095 (1972).
Kh. M. Minachev, R. V. Dmitriev, O. D. Bronnikov, V. I. Garanin, and T. A. Novruzov, Izv. Akad. Nauk SSSR, Ser. Khim., 1891 (1972).
L. G. Christer, B. V. Liengme, and W. K. Hall, Trans. Faraday Soc.,64, 1679 (1968).
J. B. Uytterhoeven, P. Jacobs, K. Makay, and R. Schoonheydt, J. Phys. Chem.,72, 1768 (1968).
Kh. M. Minachev and Ya. I. Isakov, Izv. Akad. Nauk SSSR, Ser. Khim., 943 (1968).
Ya. I. Isakov and Kh. M. Minachev, Neftekhimiya,10, 805 (1970).
Kh. M. Minachev and R. V. Dmitriev, Izv. Akad. Nauk SSSR, Ser. Khim., 124 (1968).
J. B. Uytterhoeven, R. Schoonheydt, B. V. Liengme, and W. K. Hall, J. Catalysis,13, 425 (1969).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 12, pp. 2689–2696, December, 1973.
Rights and permissions
About this article
Cite this article
Minachev, K.M., Dmitriev, R.V., Isakov, Y.I. et al. Exchange of deuterium with the hydrogen of the surface of zeolite catalysts. Russ Chem Bull 22, 2625–2630 (1973). https://doi.org/10.1007/BF00926124
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00926124