Conclusions
-
1.
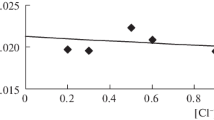
A study has been made of the effect on the hydroxyl ion concentration of the rate of ozone breakdown in aqueous solution.
-
2.
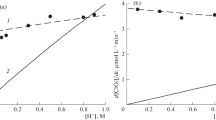
The limiting stage in this reaction is the bimolecular transfer of an electron from the hydroxyl ion to the ozone molecule.
-
3.
A kinetic description of this process has been given and rate constants for the O3-ŌH reaction calculated at four different temperatures.
Similar content being viewed by others
Literature cited
V. F. Kozhinov and I. V. Kozhinov, Ozonation of Water [in Russian], Stroiizdat (1974).
P. F. Kandzas and A. A. Mokina, Zh. Vses. Khim. 0-va.,17. 169 (1972).
S. D. Razumovskii and G. E. Zaikov, Ozone and Its Reactions with Organic Compounds [in Russian], Nauka (1974).
I. A. Kazarnovskii, D. S. Gorbenko-Germanov, and I. B. Kozlova, Izv. Akad. Nauk SSSR, Ser. Khim., 197 (1969).
I. I. Vol'nov, Peroxides, Hyperperoxides, and Ozonides of the Alkali and Alkaline-Earth Metals [in Russian], Nauka (1964).
D. S. Gorebenko-Germanov, in: Inorganic Peroxides [in Russian], Nauka (1974), p. 161.
J. Weiss, Trans. Faraday Soc.,31, 668 (1935).
M. Alder and Y. Hill, J. Am. Chem. Soc.,72, 1884 (1950).
W. Stumm, Helv. Chim. Acta,37, 773 (1954).
M. Kilpatrick and C. C. Herrik, J. Am. Chem. Soc.,78, 1784 (1962).
M. M. Raukas, é. K. Siirde, and S. P. Kyuli, Tr. Tallinsk. Politekhn. Inst.,A198, 219 (1962)
C. Hewes and R. Davison, AIChE J.,17, 141 (1971).
Yu. E. Ivanov, G. P. Nikitina, M. F. Pushlenkov, and V. G. Shumkov, Zh. Fiz. Khim.,46, 22163 (1972).
S. D. Razumovskii, Izv. Akad. Nauk SSSR, Ser. Khim., 335 (1970).
T. I. Poznyak, D. M. Lisitsyn, S. D. Razumovskii, and F. S. D'yachkovskii, Kinet. Kata.,17, 1049 (1976).
Energy of Chemical Bond Rupture. Ionization Potentials and Electron Affinities. Handbook (ed. by V. N. Kondrat'ev) [in Russian], Nauka (1974), p. 325.
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 2, pp. 289–293, February, 1979.
Rights and permissions
About this article
Cite this article
Razumovskii, S.D., Ovechkin, V.S., Konstantinova, M.L. et al. The kinetics of the reaction of ozone with hydroxyl ions in aqueous solution. Russ Chem Bull 28, 264–268 (1979). https://doi.org/10.1007/BF00925863
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00925863