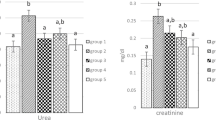
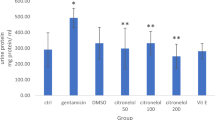
Abstract
The effect of DL α-lipoic acid on the nephrotoxic potential of gentamicin was examined. Intraperitoneal injection of gentamicin (100 mg/kg/day) to rats resulted in decreased activity of the glycolytic enzymes-hexokinase, phosphoglucoisomerase, aldolase and lactate dehydrogenase. The two gluconeogenic enzymes—glucose-6-phosphatase and fructose-1, 6-diphosphatase, the transmembrane enzymes namely the Na+, K+-ATPase, Ca2+-ATPase, Mg2+-ATPase and the brushborder enzyme alkaline phosphatase, also showed decreased activities. This decrease in the activities of ATPases and alkaline phosphatase suggests basolateral and brush border membrane damage. Decreased activity of the TCA cycle enzymes isocitrate dehydrogenase (ICDH), succinate dehydrogenase (SDH) and malate dehydrogenase (MDH), suggests a loss in mitochondrial integrity. These biochemical disturbances were effectively counteracted by lipoic acid administration. Lipoic acid administration by gastric intubation at two different concentrations (10 mg and 25 mg/kg/day) brought about an increase in the activity of the glycolytic enzymes, ATPases and the TCA cycle enzymes. The gluconeogenic enzymes however showed a further decrease in their activities at both the concentrations of lipoic acid administered. These observations shed light on the nephroprotective action of lipoic acid against experimental aminoglycoside toxicity and the protection afforded at 25 mg/kg/day of lipoic acid was noted to be higher than that at 10 mg level.
Similar content being viewed by others
References
Walter AM, Heilmeier L: In: H. Otten, M. Plempel and W. Siegenthaler (eds). Antibiotika-Fibel, Thieme, Stuttgart, 1975, p 362
GinnShelburne J, Trump B: Disorders of cell Volume regulation. Am J Pathol 53: 1041–1071, 1968
Horio M, Fukuhara Y, Orita Y, Akanishi T, Nakahara H, Moriyama T, Kumada T: Gentamicin inhibits Na+ dependent glucose transport in rabbit kidney brush border membrane vesicles. Biochim Biophys Acta 858: 153–160, 1986
Biber J, Hauser H: The role of SH-group in the concentrative transport of D-glucose into brush border membrane vesicles. FEBS Lett 108(2): 451–456, 1979
Masayuki T, Yukihiko A, Asaichi I, Seishi T: Inhibition of alkaline phosphatase activity and D-glucose uptake in rat renal brush border membrane vesicles by aminoglycosides. Biochim Biophys Acta 903: 31–36, 1987
Belly GD, Williams RJ: Effect of lipoic acid on the growth rate of young rats. Arch Biochem Biophys 55: 587–588, 1955
Jayanthi S, Jayanthi G, Varalakshmi P: Effect of DL α-lipoic acid on some carbohydrate metabolising enzymes in stone forming rats. Biochem Int 25(1): 123–136, 1991
Haugaard N, Haugaard ES: Stimulation of glucose utilisation by thiotic acid in rat diaphragm incubatedin vitro. Biochim Biophys Acta 222: 583–586, 1970
Gandhi VM, Wagh SS, Nataraj CV, Menon KKG: Lipoic acid and diabetes II. Mode of action of lipoic acid. J Bio Sci 9: 117–127, 1985
Bashan N, Burdett E, Guma A, Klip A: Effect of Thioctic acid on glucose transport. In: F.A. Gries and K. Wessel (eds). The role of antioxidants in Diabetes mellitus. Oxygen radicals and antioxidants in Diabetes. Frankfurt am Main: pmi verl-Gruppe, Germany, 1993, pp 221–229
Branstrup N, Kirk JE, Bruni C: The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerentol 238: 3280, 1963
Horrocks JE, Ward J, King J: A routine method for the determination of phosphoglucoisomerase activity in body fluid. J Clin Pathol 16: 248, 1963
King J: The trasferases—alanine and aspartate transaminases. In: D. Van (ed). Practical Clinical Enzymology, Nostrand Company Ltd, London, 1965, pp 121–138
King J: The dehydrogenases or Oxido reductases—lactate dehydrogenase. In: D. Van (ed). Practical Clinical Enzymology, Nostrand Company Ltd, London, 1965, pp 83–93
King J: The phosphohydrolases—acid and alkaline phosphatase. In: D. Van (ed). Practical Clinical Enzymology, Nostrand Company Ltd, London, 1965, pp 191–208
Gansede JM, Gancedo C: Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase fermenting and non fermenting yeasts. Arch Microbiol 76: 132, 1971
Bonting SL: Sodium—potasium activated adenosine triphosphatase and cation transport. In: E.E. Bittar (ed). Membrane ion transport. Wiley Interscience, England, 1970, pp 257–363
Ohnishi T, Suzuki T, Ozawas K: A comparative study of plasma membrane magnesium ion ATPase activities in normal, regenerating and malignant cells. Biochim Biophys Acta 684: 67–74, 1982
Hjerten S, Pan H: Purification and characterisation of two forms of a low affinity calcium ion ATPase from erythrocyte membrane. Biochim Biophys Acta 755: 457–456, 1983
Slater EC, Bonner WD: Succinate dehydrogenase. Biochem J 52: 185–196, 1952
Mehler AH, Komberg A, Crisolen S, Ochon S: J Biol Chem 174: 961–977, 1948
Sasaki T, Matsuy S, Sanae A: Effect of acetic acid concentration on the colour reaction in the O.toluidine—boric acid method for blood glucose determination. Rinsho Kagaku 1: 346, 1972
Fiske CH, Subbarow Y: The colorimetric determination of phosphorus. J Biol Chem 66: 375, 1925
Lowry OH, Rosenbrough NJ, Farr AI, Randall RJ: Protein measurement with the Folin-phenol reagent. J Biol Chem 193: 265, 1951
Kishore BK, Kallay Z, Lambricht P, Laurent G, Tulkens PM: Mechanism of protection afforded by poly aspartic acid against gentamicin induced phospholipidosis. 1. Polyaspartic acid binds gentamicin and displaces it from negatively charged phospholipid bilayersin vitro. J Pharmacol Exp Ther 255: 867–874, 1990
Smith CR, Lipsky JJ, Laskin OL, Hellmann DB, Mellits ED, Longstreth J, Lietman PS: Double-bind comparison of the nephrotoxicity and auditory ototoxicity of gentamicin and tobramycin. N Engl J Med 302: 1106–1109, 1980
Takahashi M, Aramaki Y, Inaba A, Tsuchiya S: Inhibition of alkaline phosphatase activity and D-glucose uptake in rat renal brush border membrane vesicles by aminoglycosides. Biochim Biophys Acta 903: 31–36, 1987
Singh HPP, Bouman RH: Effect of DL α-lipoic acid on the citrate concentration and phosphofructokinase activity of perfused hearts from normal and diabetic rats. Biochem Biophys Res Commun 41: 555–561, 1970
Bluementhal SA: Inhibition of gluconeogenesis in rat liver by lipoic acid—Evidence for more than one site of action. Biochem J 219: 773–780, 1984
Williams PD, Trimble ME, Crespo L, Holohan PD, Freedmon JC, Ross CR: Inhibition of renal Na+, K+-adenosine triphosphatase by gentamicin. J Pharmacol Exp Ther 231: 248–253, 1984
Kacew S: Inability of nitrendipine to protect against gentamicin nephrotoxicity in the rat. Biomed Environ Sci 2: 160–166, 1989
Weinberg MJ, Humes DH: Mechanisms of gentamicin induced dysfunction of renal cortical mitochondria. I. Effects on mitochondrial respiration. Arch Biochem Biophys 205: 222–231, 1980
Weinberg MJ, Harding GP, Humes DH: Mechanisms of gentamicin induced dysfunction of renal cortical mitochondria. II. Effects on mitochondrial monovalent cation transport. Arch Biochem Biophys 205: 232–239, 1980
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sandhya, P., Mohandass, S. & Varalakshmi, P. Role of DL α-lipoic acid in gentamicin induced nephrotoxicity. Mol Cell Biochem 145, 11–17 (1995). https://doi.org/10.1007/BF00925707
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00925707